FIGURE 2.
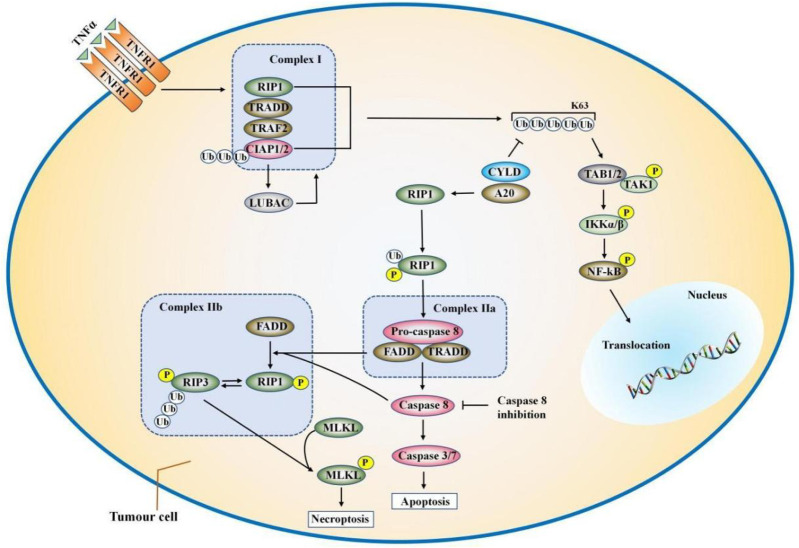
The combination of TNFα and TNFR1 can trigger a variety of signaling pathways, including NF-κB, apoptosis and necrosis. TNFα induces the formation of complex I, which is composed of RIPK1, TRADD, TRAF2/5, LUBAC, and cIAP1/2. In complex I, cIAP1/2, and LUBAC induce ubiquitination of RIPK1. The ubiquitination of the Lys63 domain of RIPK1 further promotes the formation of IKK and TAK complexes, which ultimately lead to the activation of the NF-κB pathway and cell survival. CYLD or A20 deubiquitinates RIPK1 and induced the separation of TRADD and RIPK1 from TNFR1, thereby forming complex IIa or complex IIb. FADD and pro-caspase-8 are called into TRADD and RIPK1 to form complex IIa, which activates caspase-8 through oligomerization and cleavage. When the activity of CIAPS, TAK1, NEMO is inhibited or the expression is knocked down, complex IIb is formed and caspase-8 is activated. Complex IIb contains TRADD, RIPK1, FADD, and pro-caspase-8. Then, caspase-8 induces apoptosis. When the activity of caspase-8 is blocked, such as cFLIP or the pan-caspase inhibitor zVAD-fmk, the cell will go to the necrotic pathway.