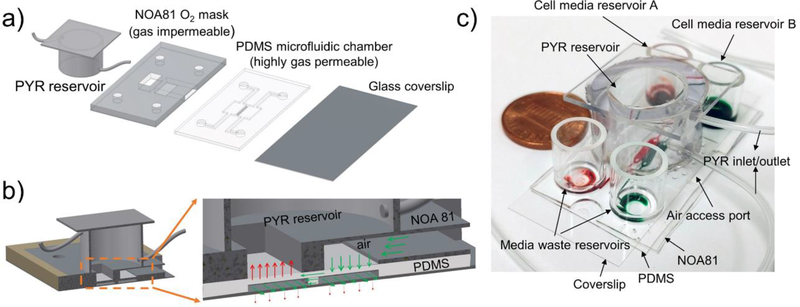
Fig. 3: A two-chamber microfluidic device for inducing hypoxia in cell co-cultures.
a) The device is comprised of a glass slide as a cell culture surface, a PDMS layer containing microfluidic chambers for co-cultivation of two groups of cells, an NOA gas-impermeable layer containing through holes and a reservoir for loading oxygen scavenging chemical. A through hole inside the NOA mask is aligned above the region of PDMS layer containing one of cell culture chambers. When oxygen scavenger, PYR, is loaded into the system it creates hypoxic conditions in one of the cell culture chambers while the neighboring chamber remains normoxic. Communication between groups of normoxic and hypoxic cells occurs via grooves (5×20×100μm, height×width×length, and 30μm spacing) molded into the bottom of the wall separating the two chambers. b) A cross-sectional view showing oxygen transport in the device. Red arrows show depletion of oxygen through a window molded in NOA layer while green arrows show diffusion of oxygen through the air access port. c) An image demonstrating that a device is compact, without gas lines and can be easily transported between different experimental stations. Devices can be loaded with oxygen scavenger PYR and placed into tissue culture incubator to create physiological conditions for cells.