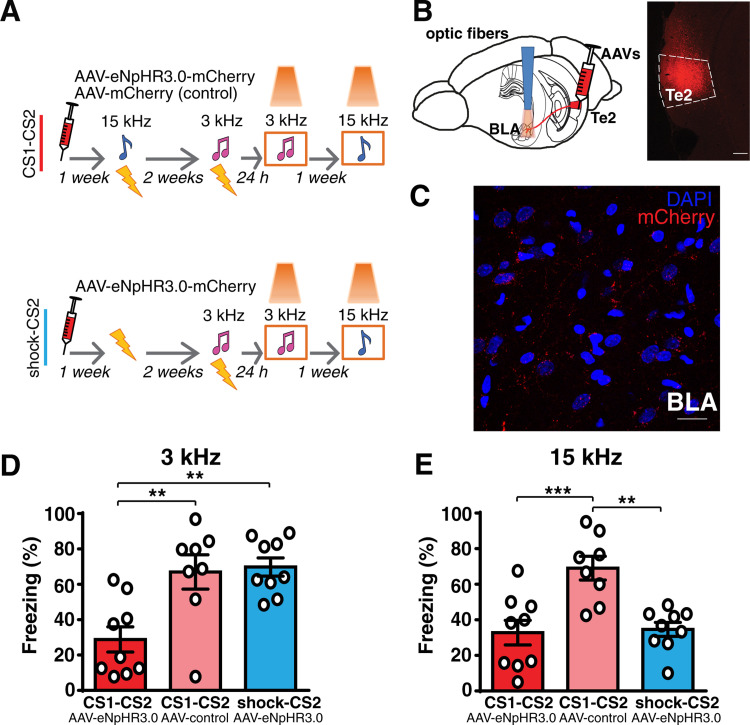
Fig 3. Prior auditory fear learning makes the Te2-to-BLA pathway necessary for the retention of new recent memories.
(A and B) The Te2 was injected with rAAV5/CamKIIa-eNpHR3.0-mCherry-WPRE or rAAV5/CamKIIa-mCherry-WPRE. Optic fibers were implanted above the BLA in order to manipulate Te2 axons terminals within the BLA. (C) Representative micrograph showing the expression of AAV-mCherry in Te2 and its terminals in the BLA. Scale bars, 300 and 20 μm, respectively. (D) Optogenetic inhibition of Te2 axon terminals in BLA affected the retention of recent fear memory only in animals that had been trained in another CS-US association (1-way ANOVA, F(2,23) = 9.85, p = 0.0008, η2 = 0.4614; Tukey: CS1-CS2 eNpHR3.0-mCherry n = 9 vs. CS1-CS2 control-mCherry n = 8, p = 0.0040; CS1-CS2 eNpHR3.0-mCherry vs. shock-CS2 eNpHR3.0-mCherry n = 9, p = 0.0016; CS1-CS2 control-mCherry vs. shock-CS2 eNpHR3.0-mCherry, p = 0.962). (E) Freezing to the tone of 15 kHz was similar between rats in which it had previously been paired to the US (remote fear memory) and in rats that had never been exposed to this tone while it was higher in rats injected with the control vector (1-way ANOVA, F(2,23) = 11.29, p = 0.0004, η2 = 0.4953; Tukey: CS1-CS2 eNpHR3.0-mCherry vs. CS1-CS2 control-mCherry, p = 0.0008; CS1-CS2 eNpHR3.0-mCherry vs. shock-CS2 eNpHR3.0-mCherry, p = 0.972; CS1-CS2 control-mCherry vs. shock-CS2 eNpHR3.0-mCherry, p = 0.0014), thus suggesting that optogenetic inhibition of the Te2-to-BLA pathway also affected the retention of remote memory. **P < 0.01, ***P < 0.001. All data are mean and SEM. The summary data for Fig 3 can be found in Supporting information in the file named S3 Data. BLA, basolateral amygdala; CS, conditioned stimuli; US, unconditioned stimulus.