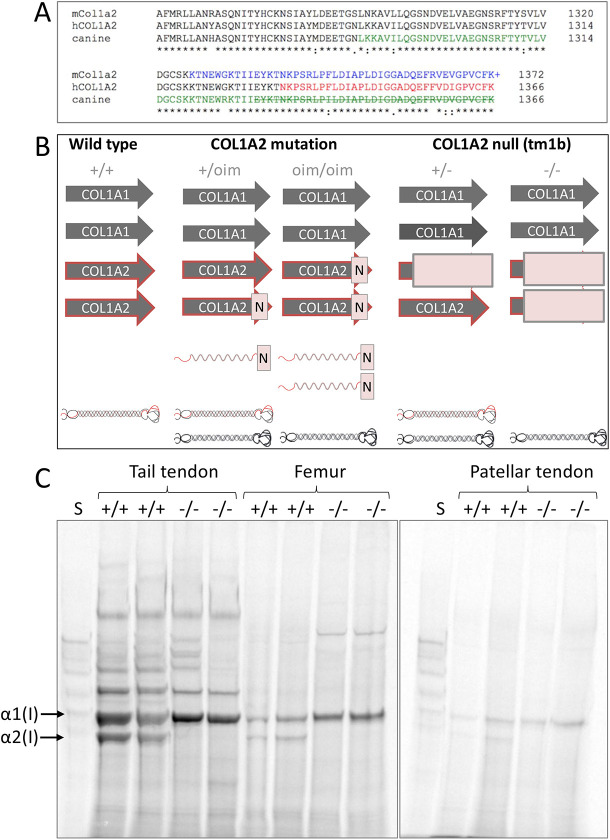
Fig. 1.
Col1a2 mutant and null alleles. (A) Amino acid sequence alignment for the final 112 residues of the mouse (mCol1a2), human (hCOL1A2) and canine α2(I) collagen chains. Colour change indicates amino acid sequence changed from that shown due to frameshift caused by the osteogenesis imperfecta murine (oim) or equivalent mutation. ‘+’ indicates reported additional amino acid (Chipman et al., 1993). Strikethrough indicates truncation. (B) Genetic differences between the oim and Col1a2 null lines with implications for collagen (I) protein synthesis. Arrows indicate COL1 genes; N indicates mutation; light-red box indicates null allele. Folded heterotrimeric proteins are indicated in black and red; homotrimers are in black only. The presence of unincorporated mutant Col1a2 allele is indicated as a red waveform with a mutation (N). (C) Tendon and bone tissue from two tm1b wild-type (+/+) and homozygote (−/−) mice were labelled with [14C]proline, and the first extracts were analysed by delayed reduction SDS-PAGE. No labelled α2(I) chain was present in tm1b homozygotes (−/−) unlike in wild-type controls (+/+), indicating that tm1b homozygotes are Col1a2 null. S, collagen standard. α1(I) and α2(I) chains are indicated.