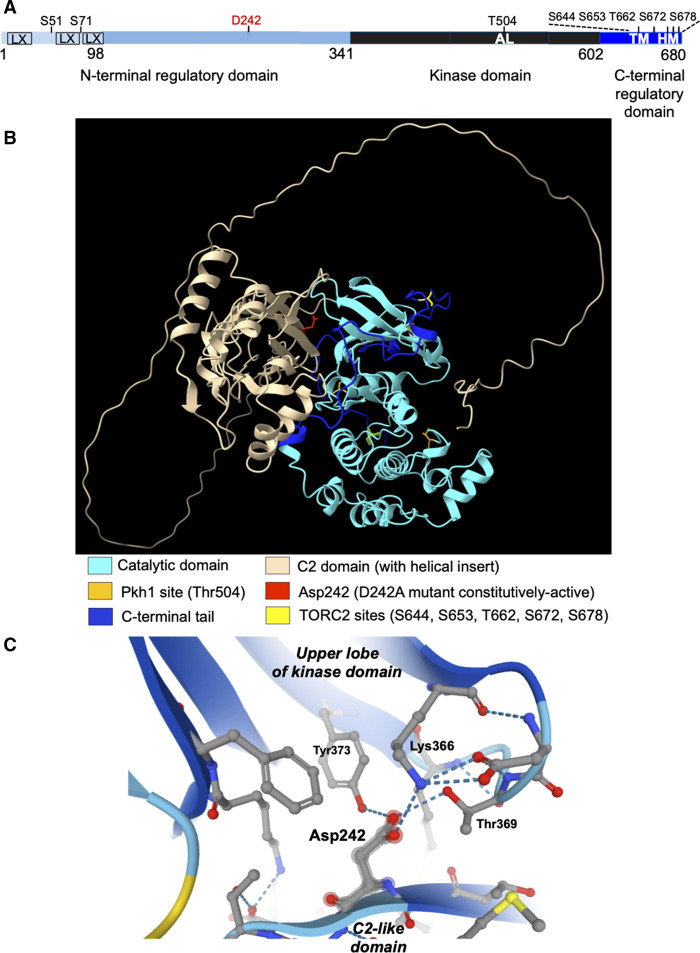
Figure 3. Ypk1 structure and regulation.
(A) Schematic depiction of Ypk1 primary structure. N-terminal regulatory domain (light blue); catalytic domain (black); and, C-terminal regulatory domain (dark blue). LX, low complexity sequence predicted by UniProt [132], SMART [133] and AlphaFold2 [43] databases; AL, Pkh1 site (T504) in activation loop; TORC2 sites: TM, classical turn motif (S644); HM, classical hydrophobic motif (T662); and additional positions (S653, S672, S678); S51 and S71, two, N-terminal sites phosphorylated by Fpk1 and paralog Fpk2. (B) Predicted three-dimensional fold of Ypk1 in the presumed ‘closed’ conformation (AF-P12688-F1-model_v2.pdb); features highlighted as in the color key shown beneath. See also Supplementary Movie S1 for animation. (C) Close-up of predicted contacts made by Asp242.