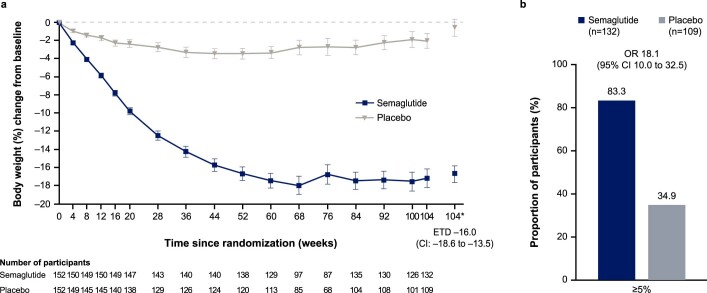
Extended Data Fig. 6. Comparison of body weight parameters for semaglutide versus placebo (trial product estimand).
(a) Observed mean percentage change from baseline in body weight over time for participants in the full analysis set during the on-treatment observation period (error bars are standard error of the mean; numbers below the panels are the number of participants contributing to the mean) and estimated treatment difference for the percentage change from baseline to week 104 in body weight based on the trial product estimand. (b) Observed proportions of participants and odds ratio for achieving weight loss of at least 5% from baseline at week 104 in the full analysis set during the on-treatment observation period, based on the trial product estimand. *Estimated means in percent. A time point is considered as on treatment if any dose of trial product has been administered within the previous 14 days. The trial product estimand assesses treatment effect assuming all participants adhered to treatment and did not receive rescue intervention. CI, confidence interval; ETD, estimated treatment difference.