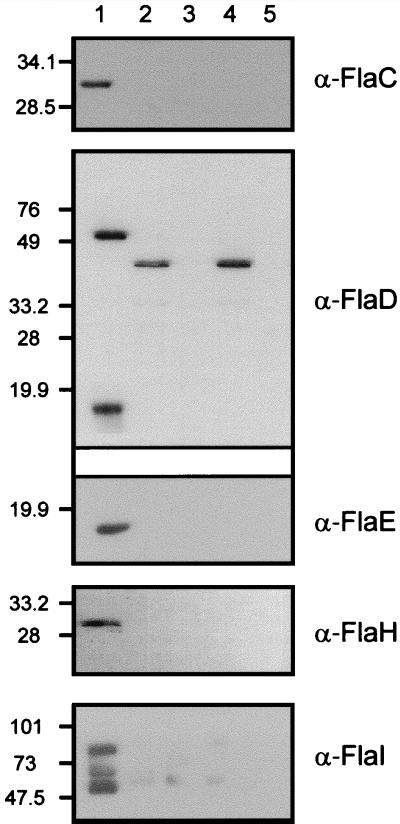
FIG. 5.
Subcellular localization of flagellar accessory proteins in M. voltae. Fractionated M. voltae cells and purified flagellar filaments were subjected to immunoblotting with polyclonal antibodies raised against purified recombinant flagellar accessory proteins. A nonflagellated M. voltae mutant, P2, which does not express any of the flagellar accessory genes due to insertional inactivation of the flagellar gene family (23), was included as a negative control. Approximately 20 μg of protein was loaded per lane. For FlaC and FlaH, 40 μg of protein was required for immunodetection. The antibodies used in the detection are indicated to the right of each panel. Lane designations apply to all the panels. Lane 1, M. voltae membrane fraction; lane 2, M. voltae cytoplasmic fraction; lane 3, P2 membrane fraction; lane 4, P2 cytoplasmic fraction; lane 5, purified M. voltae flagellar filaments. The α-FlaD antibody preparation cross-reacts with a soluble protein present in both the wild type and the P2 mutant; however FlaD localization is confirmed by the absence of a signal in the membrane fraction of the P2 mutant. Three protein species are recognized by the α-FlaI antibodies.