Table 2.
Generality of the PdL1R(OTf)/RuL2S-matched catalyst systema
| Entry | Product 3 | Yield (%) | b/l | syn/anti | erb | |
|---|---|---|---|---|---|---|
| 1c,d | 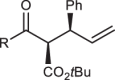 |
R = H | 82 | >95:5 | >95:5 | >99:1 (R,S) |
| 2 | R = Me | 99 | >95:5 | >95:5 | >99:1 (R,S) | |
| 3e | R = Et | 98 | >95:5 | >95:5 | >99:1 | |
| 4e | R = iPr | 91 | >95:5 | >95:5 | >99:1 | |
| 5 f | R = tBu | <5 | n.d. | n.d. | n.d. | |
| 6e,f | R = CH2OCH3 | 96 | >95:5 | >95:5 | >99:1 | |
| 7e |  |
R = H | 97 | >95:5 | >95:5 | 99:1 |
| 8e | R = p-CH3O | 95 | >95:5 | >95:5 | >99:1 | |
| 9 | R = p-Cl | 92 | >95:5 | >95:5 | >99:1 (R,S) | |
| 10e,g,h | 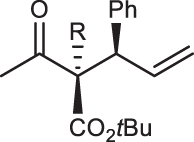 |
R = Me | 91 | >95:5 | >95:5 | >99:1 |
| 11i | R = Bn | <5 | n.d | n.d | n.d | |
| 12e,g,h | R = F | 89 | >95:5 | >95:5 | 99:1 | |
| 13e,g,h | 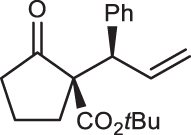 |
97 | >95:5 | >95:5 | >99:1 | |
| 14 |  |
R = o-F | 99 | >95:5 | >95:5 | >99:1 (R,S) |
| 15e | R = o-Me | 97 | >95:5 | >95:5 | 99:1 | |
| 16e | R = o-CH3O | 96 | >95:5 | >95:5 | >99:1 | |
| 17 | R = m-F | 99 | >95:5 | >95:5 | >99:1 (R,S) | |
| 18e | R = m-Me | 98 | >95:5 | >95:5 | >99:1 | |
| 19e | R = m-CH3O | 96 | >95:5 | >95:5 | >99:1 | |
| 20 | R = p-F | 99 | >95:5 | >95:5 | 99:1 (R,S) | |
| 21e | R = p-Cl | 97 | >95:5 | >95:5 | >99:1 | |
| 22e,h,j | R = p-CF3 | 87 | 93:7 | >95:5 | 99:1 | |
| 23e | R = p-Me | 98 | >95:5 | >95:5 | 99:1 | |
| 24e | R = p-CH3O | 99 | >95:5 | >95:5 | >99:1 | |
| 25 | 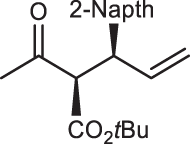 |
95 | >95:5 | >95:5 | >99:1 (R,S) | |
| 26e,g | 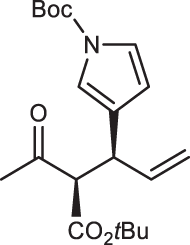 |
97 | >95:5 | >95:5 | >99:1 | |
| 27 h | 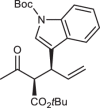 |
93 | >95:5 | >95:5 | >99:1 (R,S) | |
| 28 |  |
48 | <5:95 | — | 64:36 | |
aConditions unless otherwise specified: [1] = 500 mM; [2] = 600 mM; [PdL1R(OTf)] = [RuL2S] = 5.00 mM; 1,4-dioxane 1.00 mL; 10 °C; 24 h.
bEr; enantiomer ratio. Symbols in parentheses are absolute configuration of major stereoisomer. Details on determination are provided in the Supplementary Information.
c[1] = 500 mM; [2] = 750 mM; [PdL1R(OTf)] = 1.25 mM; [RuL2S] = 2.50 mM.
dProduct was isolated as a β-hydroxy ester after one pot-reduction using K-selectride.
eRelative and absolute configurations were not determined. Structures drawn were estimated from the structure-confirmed analogs.
fReaction time 48 h.
g[1] = 250 mM; [2] = 500 mM; [PdL1R(OTf)] = [RuL2S] = 2.50 mM.
hReaction time 72 h.
i4 mol% of PdL1R(OTf) and 4 mol% of RuL2S were used.
j2 mol% of PdL1R(OTf) and 4 mol% of RuL2S were used.
