Table 3.
Generality of the PdL1R(OTf)/RuL2R-mismatched catalyst system.a
| Entry | Product 3 | Yield (%) | b/l | syn/anti | erb | |
|---|---|---|---|---|---|---|
| 1 | 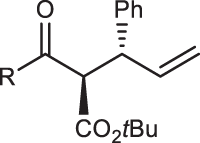 |
R = Me | 99 | >95:5 | <5:95 | >99:1 (R,R) |
| 2c | R = Et | 94 | >95:5 | 8:92 | >99:1 | |
| 3 | R = iPr | <5 | n.d. | n.d. | n.d. | |
| 4c,d | R = Ph | 93 | >95:5 | <5:95 | >99:1 | |
| 5c,d,e |  |
92 | >95:5 | 6:94 | >99:1 | |
| 6c | 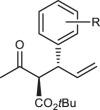 |
R = o-Me | 92 | >95:5 | 5:95 | 99:1 |
| 7 | R = m-F | 95 | >95:5 | 5:95 | >99:1 | |
| 8 | R = p-F | 91 | >95:5 | 6:94 | >99:1 | |
| 9c | R = p-Cl | 95 | >95:5 | <5:95 | >99:1 | |
| 10c | R = p-Me | 97 | >95:5 | 5:95 | >99:1 | |
aConditions unless otherwise specified: [1] = 125 mM, [2] = 150 mM; [PdL1R(OTf)] = 1.25 mM; [RuL2R] = 2.50 mM; 1,4-dioxane 4.00 mL; 10 °C; 72 h.
bEr; enantiomer ratio. Symbols in parentheses are absolute configuration of major stereoisomer. The details on determination are provided in the Supplementary Information.
cRelative and absolute configurations were not determined. Structures drawn were estimated from structure-confirmed analogs.
d96 h.
e[PdL1R(OTf)] = [RuL2R] = 2.50 mM.
