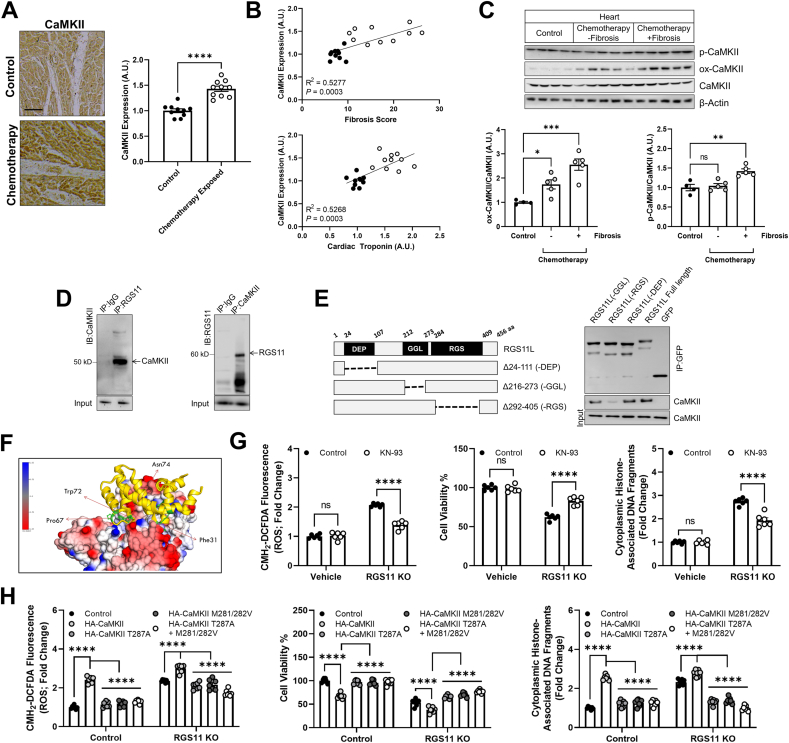
Fig. 3.
RGS11 forms a complex with CaMKII and blocks CaMKII-dependent cardiomyocyte damage. (A) Representative cardiac staining for CaMKII in a control or chemotherapy exposed patients [n = 10, scale bar = 100 μm]. (B) Correlation between CaMKII histoscores andfibrosis or cardiac troponin levels in control or chemotherapy exposed patients (n = 10). (C) Immunoblotting for phosphorylated and oxidized CaMKII protein expression in heart tissue from chemotherapy exposed patients or matched controls (n = 4–5). (D) Reciprocal co-immunoprecipitation of RGS11 and CaMKII from AC-16 cells. (E) Co-immunoprecipitation of CaMKII with RGS11 deletion constructs. (F) In silico modeling of the putative RGS11-CaMKII complex revealed key RGS11 residues predicted to support a direct interaction between RGS11 and CaMKII. (G) Control or RGS11 CRISPR KO AC-16 cells were treated with CaMKII inhibitor KN-93 (50 μM, 1 h). CM-H2-DCFDA fluorescence (total ROS), cell viability, apoptosis (cytoplasmic histone-associated DNA fragments; n = 5) were measured. (H) RGS11 KO AC-16 cells were transfected with control plasmid (vector) ± phosphorylation (T287A) or oxidation (M281/282 Vhourss)) deficient CaMKII (HA-tagged) where indicated. CM-H2-DCFDA fluorescence (total ROS), cell viability, apoptosis (cytoplasmic histone-associated DNA fragments; n = 7) were measured. β-Actin serves as a loading control for western blots. Immunoblots are accompanied by a densitometric quantification wherein expression is normalized to the corresponding control group. Data were analyzed by student's t-test or one- or two-way ANOVA with Sidak's post-hoc test. *P < 0.05, **P < 0.01,***P < 0.001, ****P < 0.0001. ns = not significant. Data are presented as mean ± SEM.