Figure 1.
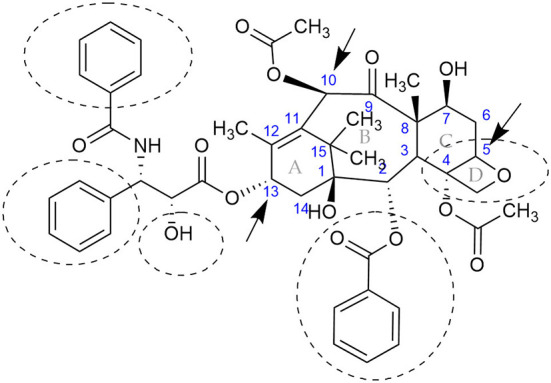
Chemical structure of the anticancer compound, paclitaxel, better known by its registered name, Taxol®. The central rings are specified with the letters A, B, C, and D, while the carbons are numbered in blue according to (Nicolaou et al., 1994). The compound is produced by Taxus spp., some fungi, actinomycetes, and, most recently, bacteria isolated from marine macroalgae. Some fungal endophytes could modify the functional groups at C-10 and C-13 (indicated by arrows at rings B and A, respectively), or modify the oxetane (ring D) and convert the paclitaxel into less active metabolites (Kingston, 1994; Hu et al., 1996; Zhang et al., 1998; Baloglu and Kingston, 1999). Functional groups that are known to be required for activity are encircled with dashed lines (Kingston, 1994).
