Key Points
Question
Is exercise capacity reduced more than 3 months after SARS-CoV-2 infection among those with long COVID-19 (LC) symptoms compared with recovered individuals without symptoms, and what patterns of limitations on cardiopulmonary exercise testing (CPET) are common?
Findings
In this systematic review and meta-analysis of 38 studies comprising 2160 participants, exercise capacity was reduced by 4.9 mL/kg/min among individuals with symptoms consistent with LC compared with individuals without symptoms more than 3 months after SARS-CoV-2 infection. Findings among individuals with exertional intolerance suggest that deconditioning, dysfunctional breathing, chronotropic incompetence, and abnormal peripheral oxygen extraction and/or use may contribute to reduced exercise capacity.
Meaning
These findings suggest that CPET may provide insight into the mechanisms for reduced exercise capacity among individuals with LC.
This systematic review and meta-analysis addresses whether adults with persistent COVID-19 symptoms more than 3 months after SARS-CoV-2 infection (long COVID-19 [LC]) have reduced exercise capacity compared with recovered individuals without symptoms and identifies potential mechanisms of LC.
Abstract
Importance
Reduced exercise capacity is commonly reported among individuals with COVID-19 symptoms more than 3 months after SARS-CoV-2 infection (long COVID-19 [LC]). Cardiopulmonary exercise testing (CPET) is the criterion standard to measure exercise capacity and identify patterns of exertional intolerance.
Objectives
To estimate the difference in exercise capacity among individuals with and without LC symptoms and characterize physiological patterns of limitations to elucidate possible mechanisms of LC.
Data Sources
A search of PubMed, EMBASE, Web of Science, preprint servers, conference abstracts, and cited references was performed on December 20, 2021, and again on May 24, 2022. A preprint search of medrxiv.org, biorxiv.org, and researchsquare.com was performed on June 9, 2022.
Study Selection
Studies of adults with SARS-CoV-2 infection more than 3 months earlier that included CPET-measured peak oxygen consumption (V̇o2) were screened independently by 2 blinded reviewers; 72 (2%) were selected for full-text review, and 35 (1%) met the inclusion criteria. An additional 3 studies were identified from preprint servers.
Data Extraction and Synthesis
Data extraction was performed by 2 independent reviewers according to the PRISMA reporting guideline. Data were pooled using random-effects models.
Main Outcomes and Measures
Difference in peak V̇o2 (in mL/kg/min) among individuals with and without persistent COVID-19 symptoms more than 3 months after SARS-CoV-2 infection.
Results
A total of 38 studies were identified that performed CPET on 2160 individuals 3 to 18 months after SARS-CoV-2 infection, including 1228 with symptoms consistent with LC. Most studies were case series of individuals with LC or cross-sectional assessments within posthospitalization cohorts. Based on a meta-analysis of 9 studies including 464 individuals with LC symptoms and 359 without symptoms, the mean peak V̇o2 was −4.9 (95% CI, −6.4 to −3.4) mL/kg/min among those with symptoms with a low degree of certainty. Deconditioning and peripheral limitations (abnormal oxygen extraction) were common, but dysfunctional breathing and chronotropic incompetence were also described. The existing literature was limited by small sample sizes, selection bias, confounding, and varying symptom definitions and CPET interpretations, resulting in high risk of bias and heterogeneity.
Conclusions and Relevance
The findings of this systematic review and meta-analysis study suggest that exercise capacity was reduced more than 3 months after SARS-CoV-2 infection among individuals with symptoms consistent with LC compared with individuals without LC symptoms, with low confidence. Potential mechanisms for exertional intolerance other than deconditioning include altered autonomic function (eg, chronotropic incompetence, dysfunctional breathing), endothelial dysfunction, and muscular or mitochondrial pathology.
Introduction
After SARS-CoV-2 infection, a substantial proportion of survivors with long COVID-19 (LC) experience persistent cardiopulmonary symptoms and exercise intolerance. Long COVID-19 may occur in 3% to 30% of individuals after SARS-CoV-2 infection,1,2,3,4,5 including nonhospitalized and vaccinated individuals,6,7 and can persist for at least 12 months.8
Cardiopulmonary exercise testing (CPET) is the criterion standard for measuring exercise capacity and aiding in the differential diagnosis of exercise limitations.9,10,11 After measuring resting cardiopulmonary parameters, participants exercise on a cycle ergometer or a treadmill with measurement of gas exchange and cardiopulmonary monitoring. Measuring oxygen consumption (V̇o2) allows for objective and reproducible determination of exercise capacity, determination of anaerobic threshold, and classification of limitations. Research CPET has provided insight into persistent symptoms after SARS,12 dyspnea in people living with HIV,13 and exercise intolerance in myalgic encephalitis and/or chronic fatigue syndrome (ME/CFS).14,15,16 Clinically, CPET is useful diagnostically for unexplained dyspnea9 and prognostically in heart failure,17 lung disease,9 and preoperative evaluations.18
Case series suggest that SARS-CoV-2 infection is associated with reduced exercise capacity.19,20 A prior narrative review of 11 studies including 581 patients21 suggested that deconditioning was a major cause of reduced exercise capacity after COVID-19 hospitalization, with literature limited by confounding and lack of controls; however, to our knowledge, no systematic review on the role of CPET in LC has been published. Whether exercise intolerance persists and is associated with LC and the pathophysiology of exertional intolerance in LC, especially among individuals who are not hospitalized, is uncertain. Therefore, the objectives of this systematic review and meta-analysis were to address whether adults with persistent COVID-19 symptoms more than 3 months after SARS-CoV-2 infection22 have reduced exercise capacity on results of CPET compared with recovered individuals without symptoms and to identify potential causal pathways for the reduced exercise capacity after SARS-CoV-2 infection.
Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline and was registered prospectively on PROSPERO (CRD42021299842) before beginning the literature search. Because the study did not constitute human participants research, the University of California, San Francisco Institutional Review Board deemed it exempt from approval and waived informed consent.
We included studies that performed CPET measurement of peak V̇o2 among adults at least 3 months after SARS-CoV-2 infection, including case series (only symptomatic individuals), cohort studies (both symptomatic and recovered individuals), and baseline data from interventional studies. Of 3256 studies that were screened by title and abstract, we selected 72 (2%) for full-text review, (1%) of which met the inclusion criteria. Studies were excluded if participants were studied less than 3 months after infection or if they did not measure V̇o2. For the first objective, we only included studies that compared individuals with and without prevalent symptoms consistent with LC at the time of CPET; for the second objective, we included studies that classified participants with exercise limitations or explored specific mechanisms of limitations.
A comprehensive search was planned with a research librarian (P.T.) to identify all studies that used CPET to evaluate exercise capacity among adults more than 3 months after SARS-CoV-2 infection, including studies published since 2020, abstracts from conference proceedings, and indexed preprints without language restrictions. We searched PubMed, EMBASE, Web of Science, and references of included studies. We additionally searched medrxiv.org, biorxiv.org, and researchsquare.com for nonindexed preprints. The search strategy included terms and synonyms for the following: COVID or SARS-CoV-2 along with cardiopulmonary exercise test, CPET or CPX or CPEX, exercise capacity, V̇o2, and anaerobic threshold tailored to each search engine (eTable 1 in the Supplement). Searches were conducted on December 20, 2021, and rerun on May 24, 2022; the preprint search was performed on June 9, 2022.
The searches were conducted by the research librarian (P.T.), with results downloaded and imported into a commercially available systemic reviews tool. After duplicates were automatically removed, 2 independent reviewers (M.S.D. and K.S.) screened each title and abstract using the systemic reviews tool and were blinded to each other’s decision regarding full-text review. Studies for which both reviewers agreed to full-text review or disagreed after reconciliation discussion underwent full-text review. After full-text review and consensus discussion, there were no disagreements regarding study inclusion. Data extraction was performed independently, in duplicate, using REDCap (eMethods and eAppendix in the Supplement). Discrepancies were corrected by the first 2 authors (M.S.D. and K.S.) reviewing the full text together.
Quality was assessed independently by 2 reviewers (M.S.D. and K.S.) using Cochrane’s Quality in Prognostic Studies tool23 to assess study populations (inclusion criteria and control group), measurement quality (CPET exercise protocols, peak V̇o2 assessment, submaximal test results, and interpretation of CPET), outcome (symptom assessment), confounding, and statistical analysis and reporting, followed by discussion and tabulation of consensus results. The GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) framework was used to guide a consensus discussion for overall outcome assessment.24
Owing to expected differences in study inclusion criteria, we used random-effects meta-analysis to estimate the mean difference in peak V̇o2 (in mL/kg/min) between those with and without prevalent LC symptoms as defined by each study using a restricted maximum likelihood variance estimator and Wald-type confidence intervals. For 2 studies that only reported median and IQR, the distance between the median and IQR upper and lower bounds were similar, so medians were taken as the mean and SD was estimated as the IQR divided by 1.3525; subgroups were combined among studies only reporting results by groups.25 Heterogeneity was assessed by examining forest plots, funnel plots, heterogeneity variance (τ2 statistic), and inconsistency (I2 statistic). Prespecified subgroup analyses by proportion hospitalized and time since infection were performed. Because of the small number of studies, tests for publication bias were not performed.
To synthesize findings for our second objective, we recorded the predominant explanatory finding for reduced exercise capacity, including deconditioning, ventilatory limitation, cardiac limitations, chronotropic incompetence, dysfunctional breathing/ventilatory inefficiency, or other limitations, and the number and proportion with each if reported. Meta-analysis was performed using Stata, version 17.1 (StataCorp LLC); 2-sided P < .05 indicated statistical significance.
Results
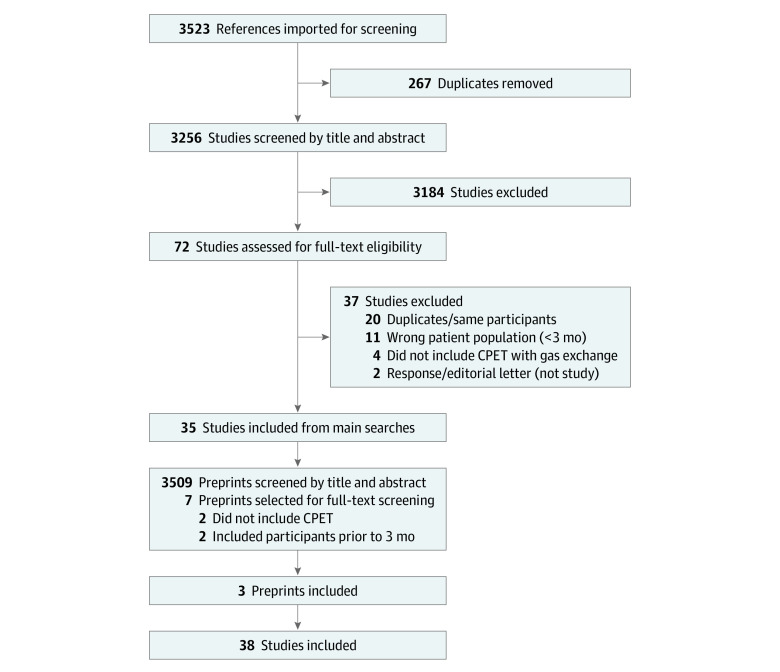
We identified 41 reports of 38 observational studies in which CPET was performed among 2160 individuals 3 to 18 months after SARS-CoV-2 infection,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66 including 1228 individuals with symptoms consistent with LC. The studies included 33 published reports, 2 conference abstracts, and 3 preprints (Figure 1). We identified 1 interventional study of cardiac rehabilitation67 with baseline CPET reported in an included study.68
Figure 1. Study Screening Diagram.
Among 3523 references identified through our primary searches, we identified 72 studies for full-text review, of which 35 met the inclusion criteria. We additionally screened 3509 preprints and identified 7 for full-text review, resulting in a total of 38 studies included. CPET indicates cardiopulmonary exercise testing.
Description of Included Studies
Table 1 lists study-level characteristics; eTable 2 and eTable 3 in the Supplement include the risk of bias assessments. Most studies (32 [84.2%]) were single-center case series of patients attending LC clinics or referred for clinical CPET (only symptomatic individuals) or cross-sectional assessments within COVID-19 recovery cohorts (with and without current symptoms). Most studies performed CPET 3 to 6 months after infection; only 1 study26 investigated individuals more than 1 year after infection. Fourteen studies (36.8%)27,30,33,35,36,44,47,49,52,55,56,62,66,68 only included individuals who were hospitalized for acute infection (median, 70% [range, 0-100%] hospitalized), and 15 studies (39.5%)30,32,39,40,41,42,43,50,51,54,55,57,59,61,64 included individuals with prevalent symptoms at CPET (median, 95% [range, 38%-100%] symptomatic).
Table 1. Summary of Studies That Included Cardiopulmonary Exercise Testing (CPET) More Than 3 Months After SARS-CoV-2 Infection.
| Source | Report type | Study design, sampling, and/or recruitment | No. with SARS-CoV-2 infection | Hospitalized with infection, No./total No. (%) | Prevalent symptoms consistent with LC, No./total No. (%) | Time since infection, da | Primary analytic comparisons |
|---|---|---|---|---|---|---|---|
| Abdallah et al,45 2021 | Research letter | Prospective cohort | 63 | 25/63 (40) | 34/49 (69) | 125 (16) | Hospitalized vs nonhospitalized45 and fatigue vs no fatigue58b |
| Alba et al,42 2021 | Peer-reviewed published report | Retrospective cohort referred for CPET from LC clinic | 18 | 3/18 (17) | 18/18 (100) | 257.5 (149-322) | PASC vs controls |
| Ambrosino et al,55 2022 | Peer-reviewed published report | Pulmonary rehabilitation after severe COVID-19 | 36 | 36/36 (100) | 36/36 (100) | NR | Normal vs reduced exercise capacity |
| Aparisi et al,47 2021 | Peer-reviewed published report | Prospective cohort post hospitalization | 70 | 70/70 (100) | 41/70 (59) | 181 (42) | Persistent dyspnea vs no residual dyspnea |
| Barbagelata et al,38 2022 | Peer-reviewed published report | Retrospective EHR review of individuals referred for clinical CPET | 200 | 39/200 (20) | 112/200 (56) | 80 (21) | LC vs no LC |
| Blumberg et al,28 2022 | Preprint | Cross-sectional study | 43 | NR | NR | 119 (24) | Vaccinated vs unvaccinated |
| Borrego Rodriguez et al,59 2021 | Conference abstract | Nonhospitalized health care workers | 57 | 0 | 57/57 (100) | >90 | Peak V̇o2 >100% vs <100% of predicted levels |
| Brown et al,44 2022 | Peer-reviewed published report | Prospective hospitalized cohort without ICU stay, myocardial injury, or comorbidities | 40 | 40/40 (100) | 20/40 (50) | Median, 106 | Self-reported normal exercise capacity vs reduced exercise capacity vs controls |
| Cassar et al,27 2021 | Peer-reviewed published report | Prospective cohort after COVID hospitalization | 42 | 42/42 (100) | NR (89% overall) | 180 (180-204) | Change in CPET from 2-3 mo to 6 mo and vs controls |
| Clavario et al,68 2021 | Peer-reviewed published report | Prospective cohort after COVID hospitalization | 200 | 200/200 (100) | 160/200 (80) | 107 (83-189) | Normal vs reduced exercise capacity |
| de Boer et al,40 2022 | Research letter | Retrospective case series of clinically referred for CPET | 50 | 5/50 (10) | 50/50 (100) | 180 (120) | Fatty acid and lactate production in PASC vs published cohorts |
| Debeaumont et al,30 2021 | Peer-reviewed published report | Retrospective case series of hospitalized patients with COVID-19 referred for CPET | 23 | 23/23 (100) | 23/23 (100) | 180 | Ward vs ICU |
| Dorelli et al,49 2021 | Research letter | Prospective cohort post hospitalization without comorbidities | 28 | 28/28 (100) | NR | 169 (28) | Exercise ventilatory inefficiency vs efficiency |
| Durstenfeld et al,26 2022 | Preprint | Prospective cohort without cardiovascular disease | 39 | 7/39 (18) | 23/39 (59) | 525 (465-552) | Cardiopulmonary symptoms vs no symptoms |
| Evers et al,54 2022 | Peer-reviewed published report | Retrospective case series of patients referred for post–COVID-19 exercise limitation or dyspnea | 30 | 21/30 (70) | 30/30 (100) | Mean, 129 | Change from CPET assessment 1 to 2 |
| Frésard et al,50 2022 | Peer-reviewed published report | Retrospective cohort of clinical CPET among patients referred for LC and persistent dyspnea | 51 | 36/51 (71) | 51/51 (100) | 119 (89) | Dysfunctional breathing vs normal breathing |
| Godinho and Freeman,61 2021 | Conference abstract | Case series of nonhospitalized patients with persistent exercise limitations | 9 | 0 | 9/9 (100) | Range, 180-360 | Descriptive |
| Jahn et al,62 2021 | Research letter | Case series of patients with severe COVID-19 pneumonitis attending posthospitalization pulmonary rehabilitation | 35 | 35/35 (100) | NR | 90 | Impaired vs normal peak V̇o2 |
| Johnsen et al,63 2021 | Peer-reviewed published report | Case series of post–COVID-19 clinic referrals for CPET for symptoms | 31 | NR (60% overall) | NR (67% overall) | 90 | Nonhospitalized vs hospitalized |
| Kersten et al,53 2021 | Peer-reviewed published report | Case series of post–COVID-19 clinic referrals for CPET if initial test results not revealing | 36 | NR (8% overall) | NR | 121 (77) | Descriptive |
| Ladlow et al,31 2022 | Peer-reviewed published report | Prospective cohort of active military personnel | 113 | 35/87 (31) | 61/87 (70) | 159 (7) | Comparisons by hospitalization and persistent symptoms compared with controls |
| Liu et al,52 2021 | Peer-reviewed published report | Prospective posthospitalization cohort | 41 | 41/41 (100) | NR | 219 (11) | Pulmonary fibrosis vs no fibrosis |
| Mancini et al,43 2021 | Peer-reviewed published report | Case series of LC clinic referrals for CPET for symptoms | 41 | 9/41 (22) | 41/41 (100) | 267 (99) | Descriptive |
| Margalit et al,37 2022 | Peer-reviewed published report | Nested case-control study within COVID recovery cohort | 141 | 14/141 (10) | 66/141 (47) | 240 (75) | Fatigue vs no significant fatigue |
| Mohr et al,41 2021 | Research letter | Case series of post–COVID-19 clinic referrals for CPET for dyspnea | 10 | 6/10 (60) | 10/10 (100) | Mean, 115 | Descriptive |
| Motiejunaite et al,46 2021 | Research letter | Prospective cohort | 114 | 104/114 (91) | 58/114 (51) | 90 (71-106) | DLCO >75 vs ≤75 |
| Moulson et al,57 2022 | Peer-reviewed published report | Case series of young athletes referred for symptoms | 21 | NR | 21/21 (100) | 90 (63) | Young symptomatic athletes vs historical controls |
| Parkes et al,64 2021 | Preprint | Retrospective cohort of patients undergoing clinical CPET | 12 | 9/12 (75) | 12/12 (100) | 182 (111) | Descriptive |
| Pleguezuelos et al,56 2021 | Peer-reviewed published report | Survivors of ARDS due to bilateral COVID-19 pneumonia requiring mechanical ventilation and tracheostomy | 15 | 15/15 (100) | NR | NR | Mechanical efficiency, peak V̇o2, and power output in patients with COVID-19 vs 3 control groups |
| Ribeiro Baptista et al,36 2022 | Peer-reviewed published report | Prospective cohort with severe COVID-19 requiring hospitalization >7 d and oxygen | 105 | 105/105 (100) | NR | 90 d after discharge | Normal vs reduced exercise capacity |
| Rinaldo et al,35,65 2021 | Research letter | Prospective cohort post hospitalization | 75 | 75/75 (100) | 39/75 (52) | 97 (26) | Normal vs reduced exercise capacity |
| Romero-Ortuno et al,32 2022 | Peer-reviewed published report | Cross-sectional study of symptomatic individuals within a prospective cohort | 80 | 14/80 (17) | 80/80 (100) | Median, 320 (range, 39-655) | Attaining >85% of predicted maximum heart rate |
| Singh et al,39 2022 | Peer-reviewed published report | Prospective cohort referred for CPET from LC clinic for unexplained exercise intolerance with negative initial findings of workup | 10 | 1/10 (10) | 10/10 (100) | 330 (30) | LC vs controls |
| Skjørten et al,33 2021 | Peer-reviewed published report | Multicenter prospective cohort post hospitalization | 156 | 156/156 (100) | 59/156 (38) | 104 (90-139) | COVID-19 vs reference population norms and no dyspnea (mMRC, 0) vs dyspnea (mMRC, 1-4) |
| Szekely et al,29 2021 | Peer-reviewed published report | Prospective cohort of individuals evaluated at the emergency department for acute COVID-19 | 71 | NR | 48/71 (68) | 91 (26) | COVID-19 vs control; asymptomatic vs symptomatic; severity of acute illness |
| Vannini et al,66 2021 | Research letter | Prospective cohort post hospitalization | 41 | 41/41 (100) | 29/41 (71) | 180 | Severity of acute illness and peak V̇o2 <80% vs ≥80% |
| von Gruenewaldt et al,51 2022 | Peer-reviewed published report | Retrospective cohort of clinical CPET | 20 | 8/20 (40) | 20/20 (100) | 217 (133-329) | Normal vs abnormal breathing pattern |
| Vonbank et al,34 2021 | Peer-reviewed published report | Prospective cohort | 100 | 18/100 (18) | NR | Median, 112 | Severity of acute infection |
Abbreviations: ARDS, adult respiratory distress syndrome; DLCO, diffusion capacity of carbon monoxide; EHR, electronic health record; ICU, intensive care unit; LC, long COVID-19; mMRC, modified Medical Research Council Dyspnea Scale; NR, not reported; PASC, postacute sequelae of COVID-19; V̇o2, oxygen consumption.
Typically defined as the time from symptom onset or positive results of polymerase chain reaction testing; some studies used the date of admission or date of hospital discharge. Unless indicated otherwise, data are presented as mean (SD) or median (IQR). Pleguezuelos et al56 reported time since hospital discharge and mean hospitalization of 23 days but not time from infection to hospitalization; therefore, it was unclear whether infection occurred more than 3 months previously. We identified an additional study by Ladlow et al69; however, because it seemed likely that there were overlapping participants, we only included the main study.
Report on same prospective cohort study.
Exercise Capacity in Symptomatic Compared With Recovered Individuals
Nine studies26,29,31,33,37,38,44,45,47,58 included both individuals with prevalent symptoms (n = 464) and recovered individuals without prevalent symptoms (n = 359) (Table 2), with the risk of bias rated for each study in eTable 2 in the Supplement and overall quality in the eResults in the Supplement. Because definitions of LC and postacute sequelae of COVID-19 have evolved, studies used different symptom definitions, mostly based on prevalent symptoms at CPET (dyspnea, fatigue, or exertional intolerance). From meta-analyses of these 9 studies, mean peak V̇o2 is estimated to be –4.9 (95% CI, –6.4 to –3.4) mL/kg/min among individuals with symptoms (P < .001) (Figure 2). The I2 statistic and funnel plot (eFigure 1 in the Supplement) suggest moderate heterogeneity. Two studies that did not find a statistically significant difference in symptom prevalence by reduced or preserved peak V̇o268 or by association between improvement in peak V̇o2 and symptoms27 were excluded for not reporting peak V̇o2 by symptoms.
Table 2. Studies Reporting Peak Oxygen Consumption (V̇o2) Among Individuals With and Without Prevalent Symptoms Consistent With Long COVID-19 (LC) After SARS-CoV-2 Infectiona.
| Source | Definition of LC symptoms | Age, y | Sex, No. (%) | BMI | Hospitalized, No. (%) | Time after infection, d | Participants with LC symptoms | Participants with no LC symptoms | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | No. | Peak V̇o2, mL/kg/min | Peak V̇o2, % predicted | No. | Peak V̇o2, mL/kg/min | Peak V̇o2, % predicted | ||||||
| Aparisi et al,47 2021 | Persistent dyspnea vs no dyspnea | 55 (12) | 45 (64) | 25 (36) | 27 (5) | 70 (100) | 181 (42) | 41 | 17.8 (15.8-21.2) | 77.8 (64.0-92.5) | 29 | 22.8 (18.8-27.7) | 99 (88-105) |
| Barbagelata et al,38 2022 | Dyspnea or fatigue persisting >45 d after onset | 49 (14) | 98 (49) | 102 (51) | 26 (6) | 39 (20) | 80 (21) | 112 | 25.8 (8.1) | 89.7 (19.9) | 88 | 28.8 (9.6) | 92.9 (18.7) |
| Brown et al,44 2022 | Self-reported reduced exercise capacity | 52 | 22 (55) | 18 (45) | 28 | 40 (100) | 106 | 20 | 14.9 (13.1-16.2) | NR | 20 | 19.1 (15.4-23.7) | NR |
| Durstenfeld et al,26 2022 | Chest pain, dyspnea, palpitations, or fatigue | 52 (42-61) | 18 (39) | 28 (61) | 30 | 7 (18) | 526 (464-553) | 23 | 21.2 (8.2) | 89 (23) | 16 | 28.8 (7.7) | 111 (20) |
| Ladlow et al,31 2022 | ≥1 Symptom | 39 | 13 (15) | 74 (85) | 29 | 35 (31) | 159 (7) | 61 | 32.4 (6.7) | NR | 26 | 40.7 (8.9) | NR |
| Margalit et al,37 2022 | Fatigue | 47 (13) | 83 (59) | 58 (41) | 28 (5) | 14 (14) | 240 (75) | 66 | 27.7 (7.5) | 96.1 (18.3) | 75 | 30.7 (7.5) | 99.6 (17.4) |
| Schaeffer et al,58 2022 | Fatigue vs no fatigue | 48 | 23 (47) | 26 (53) | 29 | 25 (40) | 125 (16) | 34 | 19.9 (7.1) | 74 (20) | 15 | 24.4 (6.7) | 81 (17) |
| Skjørten et al,33 2021 | Dyspnea, mMRC, 1-4 vs 0 | 56 | 60 (38) | 96 (62) | 28 (5) | 156 (100) | 104 (90-139) | 59 | 23.6 (7.9) | NR | 67 | 31.9 (9.3) | NR |
| Szekely et al,29 2021 | Persistent fatigue, dyspnea, muscle weakness, or pain | 53 (16) | 24 (34) | 47 (66) | 28 (6) | NRb | 91 (26) | 48 | 18.8 (6.3) and 1.5 (0.5) L/min |
NR | 23 | 21.3 (6.3) and 1.7 (0.5) L/min | NR |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); mMRC, modified Medical Research Council Dyspnea Scale; NR, not reported.
Unless indicated otherwise, data are presented as mean (SD) or median (IQR). Cells with single values report the mean; SDs were only reported by subgroup.
All patients were evaluated in the emergency department, but the number or percentage admitted was not reported.
Figure 2. Meta-analysis of Peak Oxygen Consumption (V̇o2) Among Studies Comparing Patients With and Without Long COVID-19 (LC) Symptoms.
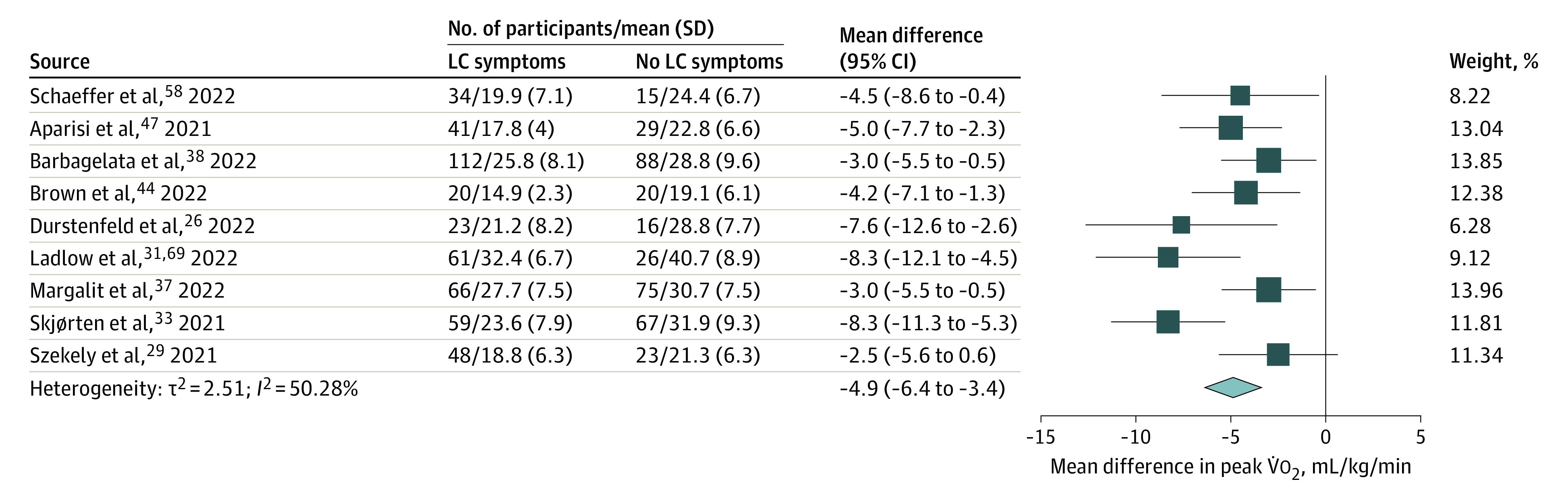
By random-effects meta-analysis of 9 studies that included 464 individuals with LC symptoms and 359 individuals without LC symptoms (as defined by each study), the mean difference in peak V̇o2 was −4.9 (95% CI, −6.4 to −3.4) mL/kg/min.
Clinical and methodologic variability likely contribute to heterogeneity. Clinical variability may result from the spectrum of LC severity and symptoms.70 Most studies recruited before widespread vaccination, but 1 study28 reported lower peak V̇o2 among unvaccinated compared with vaccinated individuals. In addition, methodologic variability in the definition of LC26,29 and CPET exercise modality (treadmill, upright cycle ergometer, or supine cycle ergometer) may also contribute. Most studies suggest higher acuity during acute infection (intensive care unit–treated vs hospitalized vs nonhospitalized patients) is associated with worse exercise capacity,30,31,32,33,34 although this is not a universal finding.35,36 Subgroup analyses by proportion hospitalized or time after SARS-CoV-2 infection were not significantly different compared with the overall result (eResults in the Supplement).
Residual confounding may also contribute to heterogeneity. Age, sex, body mass index, and prior fitness are highly associated with peak V̇o2 and likely associated with LC, but few studies addressed confounding. Margalit et al37 reported that age, sex, pre–COVID-19 fitness, body mass index, and reduction in exercise time per week were similar among those with and without LC. Barbagaleta et al38 adjusted for sex, cardiovascular history, use of β-blockers, and use of aspirin but not body mass index or age, and mean estimated peak V̇o2 was 3.2 (95% CI, 0.9-5.5) mL/min/kg lower in LC. Durstenfeld et al26 estimated that mean peak V̇o2 was 5.9 (95% CI, 2.3-9.6) mL/kg/min lower in LC adjusted for age, sex, body mass index, time since infection, and hospitalization.
Patterns of Reduced Exercise Capacity
We included 37 studies with at least 714 people with reduced exercise capacity that classified patterns or limitations or investigated specific mechanisms (Table 3).26,27,29,30,31,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,68,69 Nearly all studies defined reduced exercise capacity as less than 80% or less than 85% of predicted levels. Approaches to CPET interpretation may differ, but few studies reported using specific guidelines or algorithms or their classification approach, so notable differences emerge between studies even with similar objective findings.
Table 3. Patterns of Limitations Among Individuals With Reduced Exercise Capacity More Than 3 Months After SARS-CoV-2 Infection.
| Source | Reduced exercise capacity, No. (%) | Pattern of limitationsa | Other mechanisms | |||||
|---|---|---|---|---|---|---|---|---|
| Deconditioning | Peripheral | Cardiac | Ventilatory | Chronotropic incompetence | Dysfunctional breathing and/or ventilatory inefficacy | |||
| Abdallah et al,45 2021 | 41 (65) | Other identified | Other identified | NR | NR | Primary | NR | NR |
| Alba et al,42 2021 | 6 (33) | NA | Other identified | Other identified | Not identified | NR | NR | NR |
| Ambrosino et al,55 2022 | 28 (78) | Other identified | Other identified | NR | NR | NR | Other identified | Endothelial dysfunction |
| Aparisi et al,47 2021 | NR | NR | NA | NR | NR | NR | Primary | NA |
| Barbagelata et al,38 2022 | 39 (35) | NR | Other identified | Primary | Other identified | NR | NR | NR |
| Borrego Rodriguez et al,59 2021 | 32 (56) | Primary | Other identified | NA | Not identified | NR | NR | NR |
| Brown et al,44 2022 | 20 (50) | NR | Not identified | Primary | Not identified | NR | NR | Preload failure |
| Cassar et al,27 2021 | 6 (19) | Primary | Other identified | Not identified | Not identified | NR | NR | Symptom limitation (submaximal tests) |
| Clavario et al,68 2021 | 99 (50) | Other identified | Other identified | Other identified | Other identified | NR | NR | NR |
| de Boer et al,40 2022 | 16 (32) | NR | Primary | Other identified | Not identified | NR | NR | Altered metabolism |
| Debeaumont et al,30 2021 | 12 (52) | Other identified | NR | NR | Not identified | NR | Primary | NR |
| Dorelli et al,49 2021 | NR | NR | NR | NR | NR | NR | Primary | NR |
| Durstenfeld et al,26 2022 | 15 (38) | Other identified | NA | Other identified | Not identified | Primary | NR | NR |
| Evers et al,54 2022 | 11 (37) | NR | Primary | NR | NR | NR | NR | NR |
| Frésard et al,50 2022 | NR | NR | Other identified | NR | Primary (s) | NR | Primary (m) | NR |
| Godinho and Freeman,61 2021 | 5 (50) | Primary | Other identified | NR | Not identified | NR | NR | NR |
| Jahn et al,62 2021 | 19 (54) | Primary | NR | Other identified | Other identified | NR | NR | NR |
| Johnsen et al,63 2021 | 16 (52) | Primary | NR | NR | Other identified | NR | NR | NR |
| Kersten et al,53 2021 | 17 (55) | Other identified | NR | Other identified | Other identified | NR | NR | Pulmonary vascular |
| Ladlow et al,31 2022 | 4 (7) | Other identified (s) | Other identified (m) | NR | NR | NR | Other identified (m) | NR |
| Liu et al,52 2021 | NR | NR | NR | NR | NR | NR | Other identified | Pulmonary fibrosis |
| Mancini et al,43 2021 | 24 (59) | NR | Other identified | Other identified | Not identified | NR | Primary | Preload failure, pulmonary hypertension |
| Margalit et al,37 2022 | NR | NR | NR | NR | NR | Primary | NR | NR |
| Mohr et al,41 2021 | 8 (80) | NR | Primary | Other identified | Other identified | NR | NR | Critical illness polyneuropathy |
| Motiejunaite et al,46 2021 | 86 (75) | Primary | NR | Not identified | Other identified | NR | Primary | “Lack of motivation” (submaximal tests) |
| Moulson et al,57 2022 | 3 (14) | NR | NR | Not identified | Primary | Other identified | NR | Exertional hypotension |
| Parkes et al,64 2021 | 10 (83) | Primary | NR | NR | Other identified | NR | Primary | Pulmonary vascular |
| Pleguezuelos et al,56 2021 | NR | NR | Primary | NR | NA | NR | NR | Mechanical inefficiency |
| Ribeiro Baptista et al,36 2022 | 37 (35) | Primary | NR | Not identified | Other identified | NR | NR | NR |
| Rinaldo et al,35,65 2021 | 41 (55) | Primary | Other identified | NR | Not identified | NR | NR | NR |
| Singh et al,39 2022 | NR | NR | Primary | Not identified | NR | NR | Primary | NR |
| Skjørten et al,33 2021 | 49 (31) | Primary | NR | Other identified | Other identified | NR | Other identified | NR |
| Szekely et al,29 2021 | 49 (69) | NA | Not identified | Other identified | Not identified | Primary | NR | Insufficient stroke volume increase |
| Vannini et al,66 2021 | 19 (46) | Not identified | Not identified | Other identified | Other identified | NR | NR | NR |
| von Gruenewaldt et al,51 2022 | 2 (20) | NR | NR | NR | NR | NR | Primary | NR |
| Vonbank et al,34 2021 | NR | NR | Other identified | Other identified | Other identified | NR | NR | NR |
Abbreviation: NA, not applicable; NR, not reported.
Designation (s) indicates a pattern noted among patients with severe acute illness; (m), a pattern noted among nonhospitalized patients with acute COVID-19.
Deconditioning was reported as the most prevalent pattern by 10 studies,27,33,35,36,46,59,61,62,63,64 with alterations in muscular oxygen utilization acknowledged as an alternative explanation by some. Eight studies27,33,35,36,46,62,63,64 reporting deconditioning included mostly individuals hospitalized for severe acute COVID-19. Deconditioning may be more common among those hospitalized with other patterns (peripheral, ventilatory inefficiency) predominant among nonhospitalized patients.31 Muscular and/or peripheral oxygen extraction abnormalities were also commonly reported. Distinguishing deconditioning from altered oxygen delivery, mitochondrial dysfunction, muscular pathology, and obesity can be challenging with noninvasive CPET without adjunctive testing or pre–COVID-19 CPET for comparison. Using invasive CPET, Singh et al39 found reduced peripheral oxygen extraction, and others40,41 reported alterations in metabolism and lactate production. Importantly, none of the studies that included adjunctive cardiac imaging or right heart catheterization during exercise testing attributed their findings to deconditioning.29,39,42,43,44
Cardiac limitations were uncommon, but studies with adjunctive cardiac testing identified reduced stroke volume augmentation that was likely attributable to preload failure29,43,44; Singh et al39 did not find evidence of preload failure. Five studies26,29,37,45,69 identified chronotropic incompetence as a contributor.
Although ventilatory limitations were uncommon, dysfunctional breathing, hyperventilation, or ventilatory inefficiency (V/Q mismatch) were commonly noted.43,46,47,48,49,50,51 One study each specifically reported dysautonomia,69 pulmonary fibrosis,52 pulmonary vascular limitation,53 impaired microcirculation,54 endothelial dysfunction,55 and loss of mechanical efficiency56 as the primary cause of reduced exercise capacity. Despite concerns about pulmonary thromboembolism during acute infection, pulmonary vascular limitations were uncommon.
Longitudinal Trends
Four studies27,54,57,67 performed longitudinal CPET in a subset, including 1 interventional study. Cassar et al27 reported CPET at 2 to 3 and at 6 months; median peak V̇o2 improved from 18.0 (IQR, 14.4-21.9) to 20.5 (IQR, 17.5-26.1) mL/kg/min but remained lower than that of controls (28.1 [95% CI, 22.1-34.0] mL/kg/min; P ≤ .001 for all). Evers et al54 found no change in peak V̇o2 during 3 months among 23 individuals with reduced exercise capacity who underwent repeated CPET (mean [SD] CPET 1: 86% [19%] of predicted levels; CPET 2: 85% [21%] of predicted levels; P = .55). Moulson et al57 found improved peak V̇o2 among young symptomatic athletes 5 months after the index study, which correlated with symptom resolution. Barbara et al67 found that mean (SD) peak V̇o2 improved from 17.8 (4.6) to 20.5 (4.5) mL/kg/min after 8 weeks of cardiac rehabilitation (P < .001).
Discussion
This meta-analysis and systematic review found 38 studies that reported CPET on 2160 individuals after SARS-CoV-2 infection, including 1228 with prevalent symptoms possibly consistent with LC and 714 with reduced exercise capacity. In our meta-analysis of symptomatic vs recovered individuals more than 3 months after SARS-CoV-2 infection, we found a modest but consistent effect suggesting that exercise capacity was reduced among individuals with LC, with very low certainty in the magnitude of the effect size by GRADE (eResults in the Supplement). Given the low certainty by GRADE, we identified classifications of exercise limitations without a single conclusive mechanism. Despite the large number of participants included, the overall quality of the evidence is poor owing to the small sample size of most studies, selection bias, variability in symptom ascertainment and CPET interpretation, inadequate methods to address confounding, and lack of appropriate statistical methods.
Challenges to Estimating the Association of LC With Exercise Capacity
Selection bias was a major challenge; the included studies oversampled hospitalized individuals with greater acute severity, more comorbidities, and lower baseline fitness. Hospitalization or need for intensive care during acute infection was associated with reduced peak V̇o2 and with LC,30,31,32,33,34 but most patients with LC were not hospitalized.71 Differential selection bias may occur among individuals who are hospitalized, are referred for clinical CPET, or attend CPET after joining a cohort, which may result in overestimation of the proportion of individuals with reduced exercise capacity.
Few studies addressed confounding; the most commonly used strategies included (1) reporting the percentage of predicted peak V̇o2 that implicitly adjusts for age, sex, height, and weight; (2) group matching on age, sex, and weight; and (3) excluding individuals with comorbid cardiac, pulmonary, and musculoskeletal conditions. Preinfection fitness was an unmeasured confounder in all but 1 study37; no studies had preinfection CPET to compare within-individual change. Two excluded studies among military recruits and professional athletes found reduced peak V̇o2 at 45 to 60 days after infection compared with before infection.72,73 A few studies used stepwise regression despite small sample sizes and colinear variables, resulting in exclusion of important confounders. Only 2 studies26,38 estimated an adjusted difference in peak V̇o2 between individuals with and without LC symptoms.
Using the GRADE framework, we have low confidence in our meta-analysis estimate of the difference in exercise capacity among individuals with and without LC symptoms. The included studies provided evidence of a clinically significant, mild to moderate decrease in exercise capacity among individuals with LC compared with infected individuals without LC symptoms despite different definitions of LC.
Insights Into Mechanisms of Reduced Exercise Capacity in LC
These studies should provide insight into mechanisms of LC, yet no consistent etiology of reduced exercise capacity has emerged, likely because of heterogeneity in inclusion criteria, variability in interpretation (measurement error), and the presence of multiple mechanisms of reduced exercise capacity in LC. Deconditioning, which occurs to some degree after any illness but especially during and after hospitalization, was commonly identified. On results of noninvasive CPET, peripheral mechanisms related to oxygen delivery and/or extraction due to muscular, mitochondrial, or vascular pathology can be misattributed to deconditioning. Use of invasive CPET, stress echocardiography, or stress magnetic resonance imaging allows for measurement or approximation of cardiac output, preload, pulmonary hypertension, and peripheral oxygen extraction and may therefore allow for more accurate classification. Overall, we found consistent evidence that deconditioning is not the only explanation of reduced exercise capacity in LC, especially among individuals who were not hospitalized.
Apart from peripheral mechanisms, other commonly reported patterns include (1) dysfunctional breathing or hyperventilation unexplained by baseline pulmonary function tests or findings on cross-sectional imaging, (2) chronotropic incompetence, and (3) preload failure despite normal resting cardiac function. Ventilatory, pulmonary vascular, and cardiac limitations are uncommon, suggesting that direct heart or lung damage (especially given other negative testing results) are not major drivers of exercise limitations in LC. From the diversity of interpretations, different phenotypes resulting in exertional intolerance seem more likely than a single unifying mechanism.
Autonomic dysfunction and endothelial dysfunction are possible mechanisms for these findings and could be caused by SARS-CoV-2 infection of neurons and endothelial cells, chronic inflammation, or autoimmune mechanisms. One included study found endothelial dysfunction55 and 2 suggested dysautonomia37,69 to be associated with reduced exercise capacity in LC. Dysfunctional breathing may also be a manifestation of dysautonomia.69 Autonomic nervous system and endothelial interaction may regulate peripheral vasomotor tone16; together, they may explain differences in peripheral extraction and preload failure. Small-fiber neuropathy among individuals who have LC symptoms with postural orthostatic tachycardia syndrome may be associated with reduced cerebral blood flow and postural symptoms.74,75 No published studies included comprehensive autonomic testing, endothelial testing, and CPET.
Comparison With ME/CFS
Myalgic encephalitis/chronic fatigue syndrome is associated with reduced peak V̇o2, lower ventilatory efficiency, higher perceived exertion, and lower peak heart rates,15 and chronotropic incompetence may contribute to exercise limitations.14 Alternatively, small-fiber neuropathy causing peripheral shunting reduces exercise capacity in ME/CFS.16 Postexertional malaise (PEM; recurrence or worsening of symptoms after exercise) has been reported in LC, similar to ME/CFS.76,77 The overlap between ME/CFS and LC and whether LC has similar pathophysiology to ME/CFS remain unknown.
Recommendations for CPET for LC Clinical Care and Research
Given the heterogeneity of phenotypes of LC and lack of a single mechanism, CPET is clinically useful to narrow the differential diagnosis of exertional dyspnea in LC. A CPET result within reference range without cardiopulmonary limitations will reassure some individuals with LC and increase comfort with physical activity. For those with objective limitations, identifying a cardiac or ventilatory limitation could provide clues for further diagnostic testing and treatment. Risk of PEM should be considered in evaluation of the risk-benefit ratio of CPET among individuals reporting PEM.
With regard to research, determining the prevalence of exercise intolerance requires intentional sampling. Selection of control groups requires particular attention tailored to the research question. We recommend that CPET be performed as a maximal test that allows for assessment of chronotropy except for individuals with significant PEM, with adjunctive measures as per local expertise. Careful postexertional symptom assessment, including after CPET and 2-day CPET protocols, may provide insights into PEM in individuals with LC symptoms. Correlative data with autonomical testing may provide mechanistic insights. Given high reproducibility within individuals and reduced exercise capacity among individuals with LC symptoms, CPET may be a useful objective measure to include in interventional trials for potential LC therapeutics.
Limitations
This study has some limitations. The search plan was not peer reviewed, and the search was not limited to peer-reviewed studies. We may have missed studies that met our inclusion criteria, especially recent preprints. Many included studies were case series, which contributed only to classification of exercise limitations. Because of selection bias, we could not estimate the prevalence of reduced exercise capacity. There was moderate heterogeneity in the included studies. Additionally, we cannot rule out publication bias contributing to exaggeration of effect estimates, especially because 2 excluded studies did not find an association, although we mitigated this by including preprints and conference abstracts.
Conclusions
In this meta-analysis and systematic review, we found evidence that exercise capacity is reduced after SARS-CoV-2 infection among individuals who have symptoms consistent with LC, with a low confidence in the effect size. Further research should include longitudinal assessments to understand the trajectory of exercise capacity. Interventional trials of potential therapies are urgently needed, including studies of rehabilitation to address deconditioning, as well as further mechanistic investigation into dysfunctional breathing, autonomic dysfunction, chronotropic incompetence, impaired oxygen uptake or utilization, and preload failure to identify treatments for LC.
eTable 1. Search Strategies for PubMed, Web of Science, and EMBASE
eTable 2. Quality Assessment and Potential Threats to Validity Among Studies Included in Comparison of Peak V̇o2 Among Those With and Without Symptoms >3 Months After SARS-CoV-2 Infection
eTable 3. Quality Assessment and Potential Threats to Validity Among Studies Included in Assessment of Limitations of Exercise Capacity
eMethods. Study Protocol
eAppendix. Study Findings and Quality Form
eResults. Sensitivity Analyses and GRADE Assessment
eFigure. Funnel Plot of Studies Comparing Peak V̇o2 Among People With and Without Symptoms
References
- 1.Groff D, Sun A, Ssentongo AE, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4(10):e2128568. doi: 10.1001/jamanetworkopen.2021.28568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirschtick JL, Titus AR, Slocum E, et al. Population-based estimates of post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (PASC) prevalence and characteristics. Clin Infect Dis. 2021;73(11):2055-2064. doi: 10.1093/cid/ciab408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273 618 survivors of COVID-19. PLoS Med. 2021;18(9):e1003773. doi: 10.1371/journal.pmed.1003773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Office for National Statistics . Technical article: updated estimates of the prevalence of post-acute symptoms among people with coronavirus (COVID-19) in the UK: 26 April 2020 to 1 August 2021. September 16, 2021. Accessed April 29, 2022. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/technicalarticleupdatedestimatesoftheprevalenceofpostacutesymptomsamongpeoplewithcoronaviruscovid19intheuk/26april2020to1august2021 [Google Scholar]
- 5.Yomogida K, Zhu S, Rubino F, Figueroa W, Balanji N, Holman E. Post-acute sequelae of SARS-CoV-2 infection among adults aged ≥18 years—Long Beach, California, April 1-December 10, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(37):1274-1277. doi: 10.15585/mmwr.mm7037a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie Y, Bowe B, Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun. 2021;12(1):6571. doi: 10.1038/s41467-021-26513-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28(7):1461-1467. doi: 10.1038/s41591-022-01840-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang L, Yao Q, Gu X, et al. 1-Year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398(10302):747-758. doi: 10.1016/S0140-6736(21)01755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Thoracic Society; American College of Chest Physicians . ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211-277. doi: 10.1164/rccm.167.2.211 [DOI] [PubMed] [Google Scholar]
- 10.Wasserman KHJ, Sue DY, Stringer W, Whipp BJ. Principles of Exercise Testing and Interpretation. 4th ed. Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 11.Balady GJ, Arena R, Sietsema K, et al. ; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Interdisciplinary Council on Quality of Care and Outcomes Research . Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191-225. doi: 10.1161/CIR.0b013e3181e52e69 [DOI] [PubMed] [Google Scholar]
- 12.Chan VL, Lam JY, Leung WS, Lin AW, Chu CM. Exercise limitation in survivors of severe acute respiratory syndrome (SARS). Chest. 2004;126(4):737S. doi: 10.1378/chest.126.4_MeetingAbstracts.737S [DOI] [Google Scholar]
- 13.Patterson AJ, Sarode A, Al-Kindi S, et al. Evaluation of dyspnea of unknown etiology in HIV patients with cardiopulmonary exercise testing and cardiovascular magnetic resonance imaging. J Cardiovasc Magn Reson. 2020;22(1):74. doi: 10.1186/s12968-020-00664-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davenport TE, Lehnen M, Stevens SR, VanNess JM, Stevens J, Snell CR. Chronotropic intolerance: an overlooked determinant of symptoms and activity limitation in myalgic encephalomyelitis/chronic fatigue syndrome? Front Pediatr. 2019;7:82. doi: 10.3389/fped.2019.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook DB, VanRiper S, Dougherty RJ, et al. ; MCAM Study Group . Cardiopulmonary, metabolic, and perceptual responses during exercise in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a multi-site clinical assessment of ME/CFS (MCAM) sub-study. PLoS One. 2022;17(3):e0265315. doi: 10.1371/journal.pone.0265315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joseph P, Arevalo C, Oliveira RKF, et al. Insights from invasive cardiopulmonary exercise testing of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Chest. 2021;160(2):642-651. doi: 10.1016/j.chest.2021.01.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malhotra R, Bakken K, D’Elia E, Lewis GD. Cardiopulmonary exercise testing in heart failure. JACC Heart Fail. 2016;4(8):607-616. doi: 10.1016/j.jchf.2016.03.022 [DOI] [PubMed] [Google Scholar]
- 18.Levett DZH, Jack S, Swart M, et al. ; Perioperative Exercise Testing and Training Society (POETTS) . Perioperative cardiopulmonary exercise testing (CPET): consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br J Anaesth. 2018;120(3):484-500. doi: 10.1016/j.bja.2017.10.020 [DOI] [PubMed] [Google Scholar]
- 19.Baratto C, Caravita S, Faini A, et al. Impact of COVID-19 on exercise pathophysiology: a combined cardiopulmonary and echocardiographic exercise study. J Appl Physiol (1985). 2021;130(5):1470-1478. doi: 10.1152/japplphysiol.00710.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arena R, Faghy MA. Cardiopulmonary exercise testing as a vital sign in patients recovering from COVID-19. Expert Rev Cardiovasc Ther. 2021;19(10):877-880. doi: 10.1080/14779072.2021.1985466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naeije R, Caravita S. Phenotyping long COVID. Eur Respir J. 2021;58(2):2101763. doi: 10.1183/13993003.01763-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition . A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22(4):e102-e107. doi: 10.1016/S1473-3099(21)00703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cochrane’s Quality in Prognostic Studies tool. Accessed December 16, 2021. http://methods.cochrane.org/sites/methods.cochrane.org.prognosis/files/uploads/QUIPS%20tool.pdf
- 24.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines, 3: rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401-406. doi: 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPTLT, Deeks JJ. Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3. Updated February 2022. Accessed May 12, 2022. https://training.cochrane.org/handbook/PDF/v6.3
- 26.Durstenfeld MS, Peluso MJ, Kaveti P, et al. Inflammation during early post-acute COVID-19 is associated with reduced exercise capacity and Long COVID symptoms after 1 year. medRxiv. Preprint posted online June 1, 2022. doi: 10.1101/2022.05.17.22275235 [DOI]
- 27.Cassar MP, Tunnicliffe EM, Petousi N, et al. Symptom persistence despite improvement in cardiopulmonary health—insights from longitudinal CMR, CPET and lung function testing post–COVID-19. EClinicalMedicine. 2021;41:101159. doi: 10.1016/j.eclinm.2021.101159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blumberg Y, Edelstein M, Jabal KA, et al. Protective effect of BNT162b2 vaccination on aerobic capacity following mild to moderate SARS-CoV-2 infection: a cross sectional study, Israel, March-December 2021. medRxiv. Preprint posted online January 2, 2022. doi: 10.1101/2021.12.30.21268538 [DOI] [PMC free article] [PubMed]
- 29.Szekely Y, Lichter Y, Sadon S, et al. Cardiorespiratory abnormalities in patients recovering from coronavirus disease 2019. J Am Soc Echocardiogr. 2021;34(12):1273-1284.e9. doi: 10.1016/j.echo.2021.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Debeaumont D, Boujibar F, Ferrand-Devouge E, et al. Cardiopulmonary exercise testing to assess persistent symptoms at 6 months in people with COVID-19 who survived hospitalization: a pilot study. Phys Ther. 2021;101(6):pzab099. doi: 10.1093/ptj/pzab099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ladlow P, O’Sullivan O, Bennett AN, et al. The effect of medium-term recovery status after COVID-19 illness on cardiopulmonary exercise capacity in a physically active adult population. J Appl Physiol (1985). 2022;132(6):1525-1535. doi: 10.1152/japplphysiol.00138.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero-Ortuno R, Jennings G, Xue F, Duggan E, Gormley J, Monaghan A. Predictors of submaximal exercise test attainment in adults reporting long COVID symptoms. J Clin Med. 2022;11(9):2376. doi: 10.3390/jcm11092376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skjørten I, Ankerstjerne OAW, Trebinjac D, et al. Cardiopulmonary exercise capacity and limitations 3 months after COVID-19 hospitalisation. Eur Respir J. 2021;58(2):2100996. doi: 10.1183/13993003.00996-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vonbank K, Lehmann A, Bernitzky D, et al. Predictors of prolonged cardiopulmonary exercise impairment after COVID-19 infection: a prospective observational study. Front Med (Lausanne). 2021;8:773788. doi: 10.3389/fmed.2021.773788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rinaldo RF, Mondoni M, Parazzini EM, et al. Severity does not impact on exercise capacity in COVID-19 survivors. Respir Med. 2021;187:106577. doi: 10.1016/j.rmed.2021.106577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribeiro Baptista B, d’Humières T, Schlemmer F, et al. Identification of factors impairing exercise capacity after severe COVID-19 pulmonary infection: a 3-month follow-up of prospective COVulnerability cohort. Respir Res. 2022;23(1):68. doi: 10.1186/s12931-022-01977-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Margalit I, Yelin D, Sagi M, et al. Risk factors and multidimensional assessment of long COVID fatigue: a nested case-control study. Clin Infect Dis. 2022;ciac283. doi: 10.1093/cid/ciac283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barbagelata L, Masson W, Iglesias D, et al. Cardiopulmonary exercise testing in patients with post–COVID-19 syndrome. Med Clin (Engl Ed). 2022;159(1):6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh I, Joseph P, Heerdt PM, et al. Persistent exertional intolerance after COVID-19: insights from invasive cardiopulmonary exercise testing. Chest. 2022;161(1):54-63. doi: 10.1016/j.chest.2021.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Boer E, Petrache I, Goldstein NM, et al. Decreased fatty acid oxidation and altered lactate production during exercise in patients with post-acute COVID-19 syndrome. Am J Respir Crit Care Med. 2022;205(1):126-129. doi: 10.1164/rccm.202108-1903LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohr A, Dannerbeck L, Lange TJ, et al. Cardiopulmonary exercise pattern in patients with persistent dyspnoea after recovery from COVID-19. Multidiscip Respir Med. 2021;16(1):732. doi: 10.4081/mrm.2021.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alba GA, Ziehr DR, Rouvina JN, et al. Exercise performance in patients with post-acute sequelae of SARS-CoV-2 infection compared to patients with unexplained dyspnea. EClinicalMedicine. 2021;39:101066. doi: 10.1016/j.eclinm.2021.101066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mancini DM, Brunjes DL, Lala A, Trivieri MG, Contreras JP, Natelson BH. Use of cardiopulmonary stress testing for patients with unexplained dyspnea post-coronavirus disease. JACC Heart Fail. 2021;9(12):927-937. doi: 10.1016/j.jchf.2021.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown JT, Saigal A, Karia N, et al. Ongoing exercise intolerance following COVID-19: a magnetic resonance-augmented cardiopulmonary exercise test study. J Am Heart Assoc. 2022;11(9):e024207. doi: 10.1161/JAHA.121.024207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abdallah SJ, Voduc N, Corrales-Medina VF, et al. Symptoms, pulmonary function, and functional capacity four months after COVID-19. Ann Am Thorac Soc. 2021;18(11):1912-1917. doi: 10.1513/AnnalsATS.202012-1489RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Motiejunaite J, Balagny P, Arnoult F, et al. Hyperventilation as one of the mechanisms of persistent dyspnoea in SARS-CoV-2 survivors. Eur Respir J. 2021;58(2):2101578. doi: 10.1183/13993003.01578-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aparisi Á, Ybarra-Falcón C, García-Gómez M, et al. Exercise ventilatory inefficiency in post–COVID-19 syndrome: insights from a prospective evaluation. J Clin Med. 2021;10(12):2591. doi: 10.3390/jcm10122591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crisafulli E, Dorelli G, Sartori G, Dalle Carbonare L. Exercise ventilatory inefficiency may be a relevant CPET-feature in COVID-19 survivors. Int J Cardiol. 2021;343:200. doi: 10.1016/j.ijcard.2021.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dorelli G, Braggio M, Gabbiani D, et al. ; on behalf of the Respicovid Study Investigators . Importance of cardiopulmonary exercise testing amongst subjects recovering from COVID-19. Diagnostics (Basel). 2021;11(3):507. doi: 10.3390/diagnostics11030507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frésard I, Genecand L, Altarelli M, et al. Dysfunctional breathing diagnosed by cardiopulmonary exercise testing in “long COVID” patients with persistent dyspnoea. BMJ Open Respir Res. 2022;9(1):e001126. doi: 10.1136/bmjresp-2021-001126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von Gruenewaldt A, Nylander E, Hedman K. Classification and occurrence of an abnormal breathing pattern during cardiopulmonary exercise testing in subjects with persistent symptoms following COVID-19 disease. Physiol Rep. 2022;10(4):e15197. doi: 10.14814/phy2.15197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu M, Lv F, Huang Y, Xiao K. Follow-up study of the chest CT characteristics of COVID-19 survivors seven months after recovery. Front Med (Lausanne). 2021;8:636298. doi: 10.3389/fmed.2021.636298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kersten J, Baumhardt M, Hartveg P, et al. Long COVID: distinction between organ damage and deconditioning. J Clin Med. 2021;10(17):3782. doi: 10.3390/jcm10173782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Evers G, Schulze AB, Osiaevi I, et al. Sustained impairment in cardiopulmonary exercise capacity testing in patients after COVID-19: a single center experience. Can Respir J. 2022;2022:2466789. doi: 10.1155/2022/2466789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ambrosino P, Parrella P, Formisano R, et al. Cardiopulmonary exercise performance and endothelial function in convalescent COVID-19 patients. J Clin Med. 2022;11(5):1452. doi: 10.3390/jcm11051452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pleguezuelos E, Del Carmen A, Llorensi G, et al. Severe loss of mechanical efficiency in COVID-19 patients. J Cachexia Sarcopenia Muscle. 2021;12(4):1056-1063. doi: 10.1002/jcsm.12739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moulson N, Gustus SK, Scirica C, et al. Diagnostic evaluation and cardiopulmonary exercise test findings in young athletes with persistent symptoms following COVID-19. Br J Sports Med. Published online May 18, 2022. doi: 10.1136/bjsports-2021-105157 [DOI] [PubMed] [Google Scholar]
- 58.Schaeffer MR, Cowan J, Milne KM, et al. Cardiorespiratory physiology, exertional symptoms, and psychological burden in post-COVID-19 fatigue. Respir Physiol Neurobiol. 2022;302:103898. doi: 10.1016/j.resp.2022.103898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borrego Rodriguez J, Berenguel Senen A, De Cabo Porras C, et al. Cardiopulmonary exercise test in patients with persistent dyspnea after COVID-19 disease. Eur Heart J. 2021;42(suppl 1):ehab724.2675. doi: 10.1093/eurheartj/ehab724.2675 [DOI] [Google Scholar]
- 60.Raman B, Cassar MP, Tunnicliffe EM, et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31:100683. doi: 10.1016/j.eclinm.2020.100683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Godinho L, Freeman A. Cardiopulmonary exercise testing to evaluate exercise limitation and shortness of breath in long COVID. Thorax. 2021;76(suppl 2):A19-A20. [Google Scholar]
- 62.Jahn K, Sava M, Sommer G, et al. Exercise capacity impairment after COVID-19 pneumonia is mainly caused by deconditioning. Eur Respir J. 2021;59(1):2101136. doi: 10.1183/13993003.01136-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnsen S, Sattler SM, Miskowiak KW, et al. Descriptive analysis of long COVID sequelae identified in a multidisciplinary clinic serving hospitalised and non-hospitalised patients. ERJ Open Res. 2021;7(3):00205-02021. doi: 10.1183/23120541.00205-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parkes E, Shakespeare J, Robbins T, Kyrou I, Randeva H, Ali A. Utility of cardiopulmonary exercise testing (CPET) in the post–COVID-19 context: retrospective analysis of a single centre experience. Research Square. Preprint posted online May 25, 2021. doi: 10.21203/rs.3.rs-537361/v1 [DOI]
- 65.Rinaldo RF, Mondoni M, Parazzini EM, et al. Deconditioning as main mechanism of impaired exercise response in COVID-19 survivors. Eur Respir J. 2021;58(2):2100870. doi: 10.1183/13993003.00870-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vannini L, Quijada-Fumero A, Martín MPR, Pina NC, Afonso JSH. Cardiopulmonary exercise test with stress echocardiography in COVID-19 survivors at 6 months follow-up. Eur J Intern Med. 2021;94:101-104. doi: 10.1016/j.ejim.2021.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barbara C, Clavario P, De Marzo V, et al. Effects of exercise rehabilitation in patients with long coronavirus disease 2019. Eur J Prev Cardiol. 2022;29(7):e258-e260. doi: 10.1093/eurjpc/zwac019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clavario P, De Marzo V, Lotti R, et al. Cardiopulmonary exercise testing in COVID-19 patients at 3 months follow-up. Int J Cardiol. 2021;340:113-118. doi: 10.1016/j.ijcard.2021.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ladlow P, O’Sullivan O, Houston A, et al. Dysautonomia following COVID-19 is not associated with subjective limitations or symptoms but is associated with objective functional limitations. Heart Rhythm. 2022;19(4):613-620. doi: 10.1016/j.hrthm.2021.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peluso MJ, Kelly JD, Lu S, et al. Persistence, magnitude, and patterns of postacute symptoms and quality of life following onset of SARS-CoV-2 infection: cohort description and approaches for measurement. Open Forum Infect Dis. 2021;9(2):ofab640. doi: 10.1093/ofid/ofab640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626-631. doi: 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Crameri GAG, Bielecki M, Züst R, Buehrer TW, Stanga Z, Deuel JW. Reduced maximal aerobic capacity after COVID-19 in young adult recruits, Switzerland, May 2020. Euro Surveill. 2020;25(36):2001542. doi: 10.2807/1560-7917.ES.2020.25.36.2001542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parpa K, Michaelides M. Aerobic capacity of professional soccer players before and after COVID-19 infection. Sci Rep. 2022;12(1):11850. doi: 10.1038/s41598-022-16031-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Novak P, Mukerji SS, Alabsi HS, et al. Multisystem involvement in post-acute sequelae of coronavirus disease 19. Ann Neurol. 2022;91(3):367-379. doi: 10.1002/ana.26286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oaklander AL, Mills AJ, Kelley M, et al. Peripheral neuropathy evaluations of patients with prolonged long COVID. Neurol Neuroimmunol Neuroinflamm. 2022;9(3):e1146. doi: 10.1212/NXI.0000000000001146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wright J, Astill SL, Sivan M. The relationship between physical activity and long COVID: a cross-sectional study. Int J Environ Res Public Health. 2022;19(9):5093. doi: 10.3390/ijerph19095093 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Search Strategies for PubMed, Web of Science, and EMBASE
eTable 2. Quality Assessment and Potential Threats to Validity Among Studies Included in Comparison of Peak V̇o2 Among Those With and Without Symptoms >3 Months After SARS-CoV-2 Infection
eTable 3. Quality Assessment and Potential Threats to Validity Among Studies Included in Assessment of Limitations of Exercise Capacity
eMethods. Study Protocol
eAppendix. Study Findings and Quality Form
eResults. Sensitivity Analyses and GRADE Assessment
eFigure. Funnel Plot of Studies Comparing Peak V̇o2 Among People With and Without Symptoms