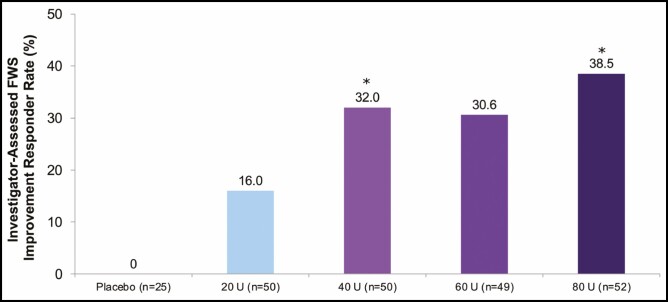
Figure 3.
Proportion of responders (subjects with a ≥1-grade FWS improvement from baseline at maximum frown) at Week 24 (primary endpoint, mITT populationa). aAll randomized and treated subjects with a baseline and at least 1 postbaseline efficacy assessment, only in the DB phase. *P < 0.05 vs onabotulinumtoxinA 20 U by the Cochran-Mantel-Haenszel test stratified by center (P value for 60 U vs 20 U not significant). DB, double-blind; FWS, Facial Wrinkle Scale; mITT, modified intent-to-treat.