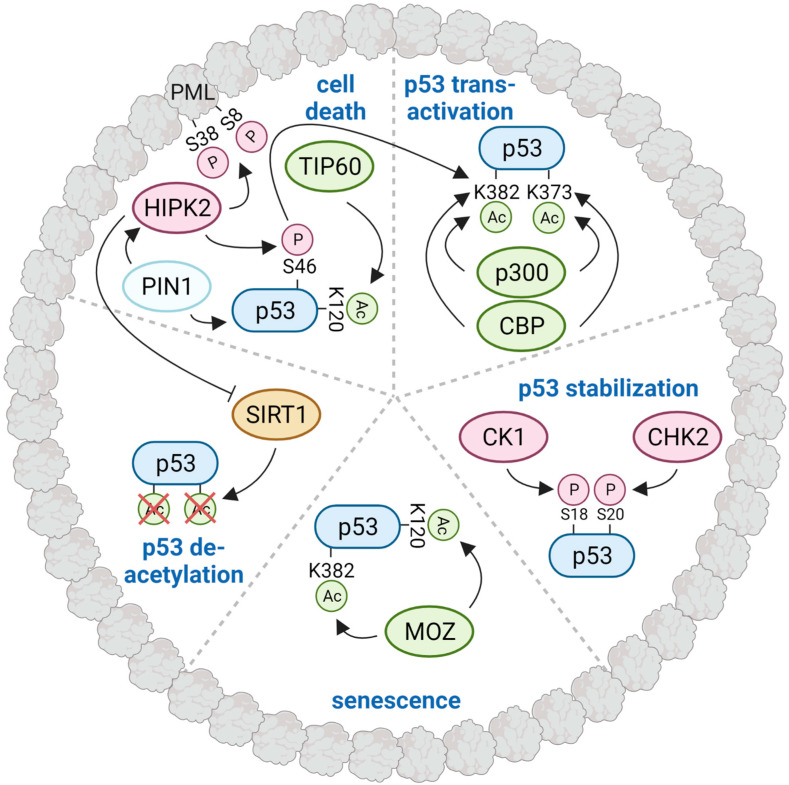
Figure 3.
Control of p53 post-translational modifications and p53 activity at PML biocondensates. Schematic representation of a mature PML NB, in which PML resides at the peripheral shell. Phosphorylation of p53 at S18 and S20 by the kinases CK1δ/ε and CHK2, respectively, is involved in p53 stabilization. Acetylation of p53 at K373/K382 by the acetyltransferases p300 and CBP results in p53 transcriptional activation of target genes. p53 acetylation is counteracted by the deacetylase SIRT1, thereby inhibiting p53-dependent apoptosis, and facilitating cell survival after DNA damage. Interaction of p53 with the acetyltransferase MOZ at PML NBs leads to p53 acetylation at K120 and K382, and, subsequently, senescence induction. p53-dependent apoptosis is stimulated by TIP60-mediated acetylation of p53 at K120 and HIPK2-catalyzed phosphorylation of S46. The p53 S46 phosphorylation mark enables interaction of p53 with the prolyl-peptidyl cis/trans isomerase, PIN1, which catalyzes p53 isomerization. This conformational change stimulates p300 and CBP-mediated p53 acetylation, thereby promoting transactivation of cell death-stimulating p53 target genes. PIN1 is also important for DNA damage-induced HIPK2 activation. Upon lethal DNA damage, HIPK2 phosphorylates SIRT1 at S682 and inhibits SIRT1 activity, thereby increasing p53 acetylation and cell death. In addition, HIPK2 also regulates PML through phosphorylation at S8 and S38, which stimulates PML SUMOylation and stabilization upon DNA damage. To what extent p53 posttranslational modifications and function are regulated by LLPS remains currently unclear.