Abstract
Introduction
Adverse events in surgery are a relevant cause of costs, disability, or death, and their incidence is a key quality indicator that plays an important role in the future of health care. In neurosurgery, little is known about the frequency of adverse events and the contribution of human error.
Research question
To determine the incidence, nature and severity of adverse events in neurosurgery, and to investigate the contribution of human error.
Material and methods
Prospective observation of all adverse events occurring at an academic neurosurgery referral center focusing on neuro-oncology, cerebrovascular and spinal surgery. All 4176 inpatients treated between September 2019 and September 2020 were included. Adverse events were recorded daily and their nature, severity and a potential contribution of human error were evaluated weekly by all senior neurosurgeons of the department.
Results
25.0% of patients had at least one adverse event. In 25.9% of these cases, the major adverse event was associated with human error, mostly with execution (18.3%) or planning (5.6%) deficiencies. 48.8% of cases with adverse events were severe (≥SAVES-v2 grade 3). Patients with multiple adverse events (8.6%) had more severe adverse events (67.6%). Adverse events were more severe in cranial than in spinal neurosurgery (57.6 vs. 39.4%).
Discussion and conclusion
Adverse events occur frequently in neurosurgery. These data can serve as benchmarks when discussing quality-based accreditation and reimbursement in upcoming health care reforms.
The high frequency of human performance deficiencies contributing to adverse events shows that there is potential to further eliminate avoidable patient harm.
Keywords: Postoperative complications; Patient harm; Patient safety; Health care reform; Outcome assessment, Health care; Quality indicators, Health Care
Highlights
-
•
Prospective observation of all patients treated at an academic neurosurgical center.
-
•
Investigation of the incidence and severity of adverse events and their relation to human error.
-
•
25.0% of patients had at least one adverse event.
-
•
Human error was involved in 25.9% of cases with adverse events.
-
•
These data provide benchmarks for tertiary care neurosurgery and health care reform.
1. Introduction
Adverse events (AEs) in surgery are a relevant cause of economic costs, disability, and death (Lawson et al., 2013; Lucas et al., 2013). In modern healthcare delivery models, such as value-based care, the goal is to achieve health outcomes that are relevant to patients while minimizing the cost of achieving them (Porter and Lee, 2013). Consequently, the incidence of AEs in surgical care has come into focus not only as a key indicator for quality assessment, but also as a potential basis for reimbursement (Gentry and Badrinath, 2017). In neurosurgery, there is ample potential for AEs that are cost-intensive and cause severe patient harm, such as unplanned returns to the operating room, or post-operative long-term neurologic deficits. However, little is known about the overall incidence of AEs in neurosurgery, as available studies are often based on retrospective analyses or focus on specific diseases or treatments only (Schipmann et al., 2019; Wong et al., 2012a, 2012b, 2012c, 2012d; Houkin et al., 2009).
The implementation of quality management and systems-based solutions in surgical care, such as checklists or standard operating procedures, has improved patient safety across all surgical disciplines, including neurosurgery (Osborne et al., 2015; Hickey et al., 2018; Leape, 1994; Han et al., 2015; Rolston and Bernstein, 2015; Westman et al., 2020). To further reduce the frequency of avoidable AEs, it is imperative to understand what causes them. Studies on human performance deficiencies in neurosurgery are scarce. The few that exist report very low rates of avoidable adverse events, but they are either based on retrospective analyses and/or focus on interventional procedures rather than the entire course of treatment (Schipmann et al., 2019; Houkin et al., 2009). A recent study identified human performance deficiencies in more than half of adverse events occurring in general surgery, suggesting that there is substantial potential for further elimination of avoidable patient harm (Suliburk et al., 2019). It is unclear how this applies to neurosurgery.
The present study aimed at a prospective observation and quantification of the incidence and severity of all AEs and specific types of AEs, as well as the incidence of human error in AEs in neurosurgery at a tertiary care university hospital.
2. Methods
2.1. Department structure
Our department provides neurosurgical service at a tertiary care university hospital. As a supra-regional referral center, we perform more than 4000 surgeries/interventions per year with a focus on neuro-oncology, cerebrovascular surgery and spine surgery. Being a national comprehensive cancer center and a level 1 spine and trauma center the complexity of the cases treated at our institution is high, as reflected by our case mix and case mix index ranking number 1 in departmental in-house comparison and number 2 in comparison with neurosurgical centers nationwide. In order to enable better comparability with different neurosurgical centers beyond the case mix index, we provide numbers of cases within different categories of complexity (based on the cost weight, i.e., a measure of individual case complexity that underlies the case mix index), stratified by diagnosis groups (Table 1 in Supplementary Material).
Our medical staff consists of eight senior board-certified neurosurgeons. As an academic teaching center, we train and educate 15 neurosurgical residents and up to three national and international surgical fellows as well as medical students. On average, we treat 90 inpatients, of which 15 are ventilated intensive care patients and 16 are intermediate care patients.
2.2. Study design
This was a prospective observation of all inpatients treated at our department within one year, starting in September 2019. All in-house AEs per case were recorded on a daily basis. The documentation of all AEs in a quality control/mortality and morbidity database had been a part of routine practice at our department before. As part of our routine, every inpatient is seen by an attending twice each day during morning and evening rounds. At the beginning of this study, it was stressed that each attending is responsible for actively screening his patients for any kind of AE every day, and for entering these AEs into our database. This included AEs occurring during surgery and AEs taking place on the regular ward, intermediate care unit and intensive care unit. We included all AEs that occurred until the patient was discharged, or until 30 days after surgery in case they had been discharged earlier and came back either as an outpatient or were readmitted.
AEs were classified along the lines of the American College of Surgeons National Surgical Quality Improvement Program 30-day outcome complication definition (ACS-NSQIP) (Lucas et al., 2013; Cohen et al., 2016), graded according to the Spine Adverse Events Severity System (SAVES-V2 grades 1–6) (Rampersaud et al., 2016) and evaluated for contribution of human error by consensus of all senior surgeons of our department during weekly discussions held after our routine mortality and morbidity conferences. They were also characterized as preoperative, intraoperative or postoperative. Due to its ubiquitous applicability to surgical patients with present or potential neurological impairment, we adopted the SAVES-V2 classification that was originally introduced for AEs in spinal surgery for cranial neurosurgery cases as well. In order to enable comparability with a broad spectrum of previous studies and across various surgical disciplines, AEs were also graded according to the Clavien-Dindo classification (Dindo et al., 2004).
Our definition of an adverse event was very liberal, including surgical and medical complications as well as events that were short-lived (e.g., two days of L5 hypesthesia after surgery for lumbar disk herniation) or that did not have an adverse effect on the patient but had the potential to cause harm (e.g., the preoperative checklist was not completed). In-house AEs were defined as those AEs that occurred during the entire hospital stay or that occurred after discharge if the patients were re-admitted within 30 days after surgery (or within 30 days after initial admission in non-operative cases). Human error was classified using a human performance deficiency (HPD) classifier (Suliburk et al., 2019). Accordingly, HPDs were assigned to five major categories: planning or problem solving, execution, rules violation, communication, and teamwork.
Our study was reviewed by the Ethics Committee of the Klinikum rechts der Isar of the Technical University of Munich. The study received a waiver of informed consent because it involves only the classification of adverse events and does not entail any treatment changes. Moreover, the documentation and discussion of adverse events by the surgeons, which has been practiced at our department for many years, was regarded as routine clinical practice.
The study was registered with the German Clinical Trials Register (DRKS00024548).
2.3. Statistical analysis
All analyses were conducted using SPSS version 25.0 (IBM Corporation, USA). The cohort was stratified into subgroups of cranial and spinal cases. Pearson's Chi-square and Mann-Whitney-U testing allowed for comparisons of categorical and ordinal variables, respectively. A two-sided P value less than 0.05 was considered statistically significant.
3. Results
3.1. Incidence of adverse events
There were 4176 patients during the study period (2258 cranial neurosurgery cases and 1918 spinal neurosurgery cases). The case mix index was 3.16 (cranial: 3.32; spinal: 2.97). 1043 (25.0%) had one AE or more (Table 1).
Table 1.
Incidence of adverse events.
| Overall | Cranial | Spinal | |
|---|---|---|---|
| Number of patients | 4176 | 2258 | 1918 |
| Case Mix Index | 3.16 | 3.32 | 2.97 |
| Cases with AE (% of patients) | 1043 (25.0) | 540 (23.9) | 503 (26.2) |
| Total number of AEs | 1611 | 895 | 716 |
| Mean number of AEs/case (range) | 1.5 (1–7) | 1.7 (1–7) | 1.4 (1–6) |
| Cases with multiple AEs (% of cases with AE) | 361 (34.6) | 215 (39.8) | 146 (29.0) |
Abbreviation: AE, Adverse Event.
Most AEs occurred during (19.6%) or after (76.3%) surgery. The most frequent AE types were urinary event (8.3% of all patients), neurological event (5.3%), unplanned return to the operating room (5.1%), iatrogenic surgical injury (4.9%), and venous thromboembolism (3.5%; Table 2).
Table 2.
Incidence of types of adverse events.
| Adverse event | Number (% of all patients) |
||
|---|---|---|---|
| Overall | Cranial | Spinal | |
| Urinary event | 346 (8.3) | 163 (7.2) | 183 (9.5) |
| Neurological event | 221 (5.3) | 166 (7.4) | 55 (2.9) |
| Unplanned return to operating room | 215 (5.1) | 127 (5.6) | 88 (4.6) |
| Iatrogenic surgical injury | 205 (4.9) | 90 (4.0) | 115 (6.0) |
| Venous thromboembolism | 145 (3.5) | 74 (3.3) | 71 (3.7) |
| Respiratory event | 122 (2.9) | 58 (2.6) | 64 (3.3) |
| Wound event | 109 (2.6) | 57 (2.5) | 52 (2.7) |
| Unexpected bleeding or transfusion | 95 (2.3) | 67 (3.0) | 28 (1.5) |
| Death | 59 (1.4) | 39 (1.7) | 20 (1.0) |
| Cardiac event | 30 (0.7) | 12 (0.5) | 18 (0.9) |
| Sepsis or septic shock | 18 (0.4) | 10 (0.4) | 8 (0.4) |
| Diagnostic failure | 11 (0.3) | 6 (0.3) | 5 (0.3) |
| Othera | 15 (0.4) | 9 (0.4) | 6 (0.3) |
Incidence of different types of adverse events (number of cases with different types of adverse events recorded within 1 year at our institution). Note that there can be multiple adverse events per case, sometimes of the same type.
Other: Types not attributable to any other category.
3.2. Multiple adverse events
The total number of AEs was 1611. Multiple AEs occurred in 8.6% of cases (up to 7 per case). The mean number of AEs per case was 1.5 (median: 1.0). The odds ratio for another AE after the first was 1.60 (1.38–1.85).
3.3. Human error in adverse events
More than one in four of the cases with AEs were associated with human error (25.9%; Table 3). The most frequent class of human performance deficiency (HPD) was execution (18.3%; e.g., screw malpositions requiring intraoperative revision in spinal surgery, or vascular injuries leading to ischemia in cranial tumor surgery). Another relevant HPD was planning or problem solving (5.6%; e.g., a suboptimally placed craniotomy, or oversight of contralateral foramen stenosis induced by instrumentation). Rules violation accounted for 1.7% of AE cases. There were hardly any communication or teamwork deficiencies contributing to AEs.
Table 3.
Incidence of human error in adverse events.
| Classification of Human Error | Number (% of cases with AE) |
||
|---|---|---|---|
| Overall | Cranial | Spinal | |
| Total | 270 (25.9) | 141 (26.1) | 129 (25.6) |
| I - Planning or problem solvinga | 58 (5.6) | 36 (6.7) | 22 (4.4) |
| II – Executionb | 191 (18.3) | 92 (17.0) | 99 (19.7) |
| III - Rules violationc | 18 (1.7) | 11 (2.0) | 7 (1.4) |
| IV – Communicationd | 1 (0.1) | 1 (0.2) | 0 (0.0) |
| V – Teamworke | 2 (0.2) | 1 (0.2) | 1 (0.2) |
Incidence of different types of human error in cases with adverse events, classified according to Suliburk and colleagues.
Abbreviation: AE, Adverse Event.
includes guideline or protocol misapplications, knowledge deficits, and cognitive bias.
includes lack of recognition, lack of attention, memory lapse, and technical errors.
includes ignoring routine or cutting corners, optimizing or personal gain, and situational or time pressure.
includes absent, assumed and misinterpreted communication.
includes ill-defined roles or lack of leadership, lack of group expertise, and failure to evaluate progress.
The majority (56.0%) of HPDs occurred during surgery; 35.6% were postoperative, and 8.4% were preoperative. These occurrence rates each differed significantly from AEs that were unrelated to human error, where 89.6% occurred postoperatively (P < .001), 5.6% preoperatively (P = .034) and only 4.8% during surgery (P < .001).
Cases with AEs related to human error were more likely to have multiple AEs than those without contribution of human error (43.7% vs. 31.4%, P < .001).
3.4. Severity of adverse events
51.2% of AEs had low severity (SAVES-V2 grades 1 and 2), corresponding to 12.8% of inpatients (Table 4). The incidence of severe AEs was substantial: 4.8% of inpatients had AEs that were either grade 4 (2.1%), 5 (1.3%) or 6 (1.4%, corresponding to mortality).
Table 4.
Severity of adverse events.
| Severity (SAVES-V2 grade) | Number (% of all patients) |
||
|---|---|---|---|
| Overall | Cranial | Spinal | |
| I - No treatment, no adverse effect | 30 (0.7) | 11 (0.5) | 19 (1.0) |
| II - Treatment minor invasive/simple, no long-term effect | 504 (12.1) | 218 (9.7) | 286 (14.9) |
| III - Treatment invasive/complex, adverse effect most likely temporary (<6 months) | 309 (7.4) | 172 (7.6) | 137 (7.1) |
| IV - Treatment invasive/complex, adverse effect most likely prolonged (>6 months) | 87 (2.1) | 62 (2.7) | 25 (1.3) |
| V - Significant neural injury or serious life-threatening event | 54 (1.3) | 38 (1.7) | 16 (0.8) |
| VI - AE resulting in death | 59 (1.4) | 39 (1.7) | 20 (1.0) |
Incidence of cases with adverse events, graded by severity according to the Spinal Adverse Events Severity System, version 2 (SAVES-V2). The most severe adverse event defined the grade in cases with multiple adverse events.
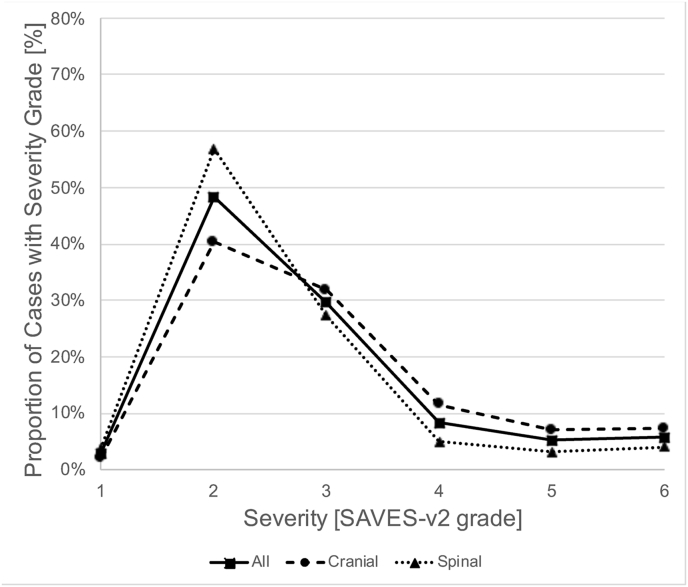
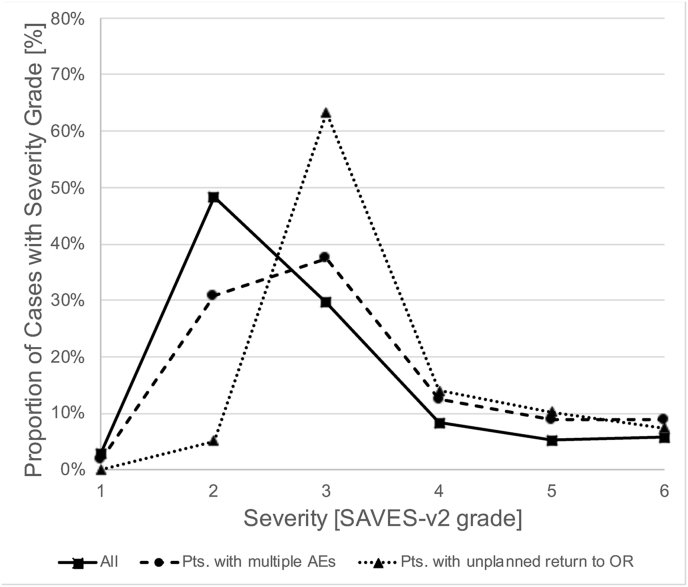
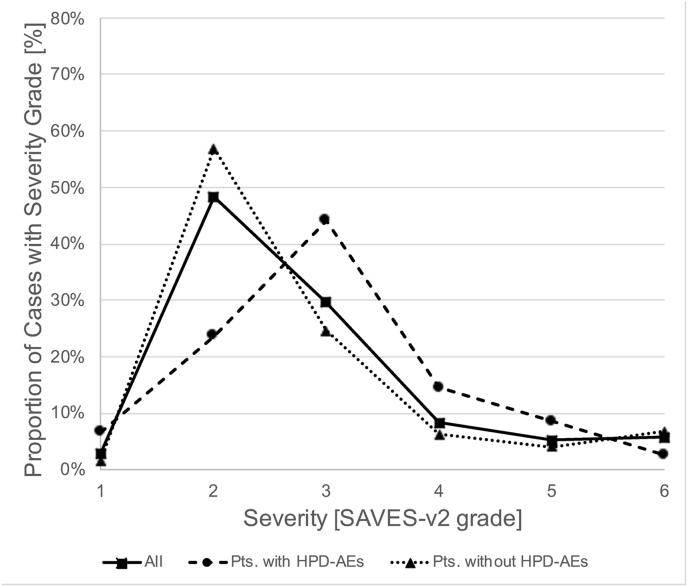
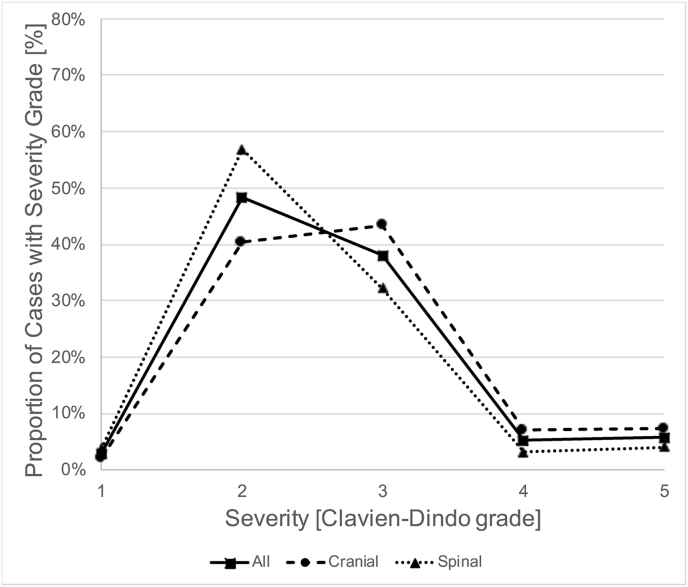
Specific subgroups of patients had different proportions of AE severity grades (Fig. 1). AEs in cranial neurosurgery were more severe than in spinal neurosurgery (P < .001, Mann-Whitney-U test; 57.6% vs. 39.4% with SAVES-V2 grade 3 or higher; Fig. 1A), and patients with multiple AEs (67.6% with SAVES-V2 grade 3 or higher; P < .001) or an unplanned return to the operating room (94.9%) had especially severe AEs (P < .001; Fig. 1B). Cases with AEs that were related to human error had more severe AEs than those that were not (P < .001; 69.6% vs. 41.5% with SAVES-V2 grade 3 or higher; Fig. 1C). Of note, grading of AE severity according to the Clavien-Dindo classification yielded a severity distribution that was similar to the SAVES-V2 grading for both cranial and spinal cases (Fig. 1D).
Fig. 1.
Severity of Adverse Events. Fig. 1A shows the relative frequency of AE severity grades according to the SAVES-V2 classification for all patients with AEs (squares and straight line), cranial neurosurgery patients (circles and dashed line) and spinal surgery patients (triangles and dotted line). Note that AEs are more severe (grades 3–6) in cranial neurosurgery compared to spinal surgery cases. Fig. 1 B shows that there are more severe AEs in patients with multiple AEs (circles, dashed line) and in patients with an unplanned return to the OR (triangles, dotted line). While the latter had the most grade 3, 4 and 5 AEs, the former had the highest fatality rate (grade 6). Fig. 1 C illustrates that in patients with AEs related to human performance deficiencies (HPD-AEs; circles, dashed line), there was a shift towards more severe AEs (grades 3–5) compared to AE-cases without HPDs (triangles, dotted line). This difference diminished with increasing severity. Of note, the mortality (grade 6) was lower in cases with HPD-AEs. Fig. 1 D shows the relative frequency of AE severity grades according to the Clavien-Dindo classification, analogous to Fig. 1 A. The distribution of AE severity is similar to that based on the SAVES-v2 classification. Abbreviations: SAVES, Spine Adverse Events Severity System; AE, Adverse Event; OR, operating room.
3.5. Cranial vs. spinal neurosurgery
Apart from AE severity, there were further differences between cranial and spinal neurosurgery. The incidence of types of AE differed (Table 2): e.g., there were more urinary events and iatrogenic surgical injuries in spinal surgery, and more neurological events, unexpected bleeding and deaths in cranial neurosurgery. In cranial cases, the odds ratio for another AE after the first was 2.11 (range: 1.73–2.58), whereas in spinal cases, the odds ratio was 1.16 (0.93–1.44). Preoperative AEs were more frequent in cranial neurosurgery (8.3% vs. 4.6% of AE-cases, P = .014), whereas intraoperative AEs occurred more often in spinal neurosurgery (23.3% vs. 16.1%, P = .004). We found no statistically significant difference between rates of postoperative AEs (cranial: 77.6% vs. spinal: 75.0%; P = .316).
4. Discussion
4.1. The incidence of adverse events in cranial neurosurgery and spinal surgery is high
We found that AEs occur frequently in cranial neurosurgery and spinal surgery. One in every four patients experienced at least one AE, which is high if compared to previous estimates of the overall AE rate in general surgery that was reported to be about 5% (Suliburk et al., 2019; Vincent et al., 2001; Gawande et al., 1999). However, the high incidence in our study is not surprising given that our data was collected prospectively, on a daily basis, until discharge of the patient, and strictly without interruption for an entire year. Thus, even though we cannot exclude underreporting (e.g., in cases when single team members decided not to report a (near) mistake that remained without consequences and could not have been noticed by others), we are confident that we detected virtually all AEs that occurred. Moreover, we intentionally set a low threshold for classifying unplanned events as adverse. Finally, as mentioned, our department's case load is high with an above average proportion of surgically and medically complex patients, which per se entails a higher incidence of AEs. At the same time, this represents the major limitation of our study: our findings are not generalizable to any neurosurgical service, but rather specific to cranial and/or spinal surgery services providing the same level of care and a comparable spectrum. To enable better comparability with different centers, we describe our departmental structure and case mix in detail (cf. Methods and Supplementary Material). Taking this into account, our data can serve as a benchmark for the incidence of AEs in cranial neurosurgery and spinal surgery at academic tertiary care centers.
4.2. Not all adverse events are necessarily unexpected or even unwanted
There are expectable AEs that occur as part of the natural course of the underlying disease regardless of treatment. For example, a patient with a severe head trauma arriving at the hospital after several hours with bilateral mydriasis and a CT angiogram that shows no perfusion will die inevitably. In this study, these events were rare (eleven cranial neurosurgery cases, corresponding to 2.0% of all cases with AEs; no spinal surgery case).
In addition, there is a different type of expectable AE that is treatment-related. We found that there is a considerable number of cranial and spinal neurosurgery cases involving surgery that is beneficial to the patient, but almost inevitably entails AEs (cranial neurosurgery: 28 cases, corresponding to 5.2% of cases with AEs; spinal surgery: 8 cases, corresponding to 1.6% of cases with AEs). For example, the complete resection of a pituitary adenoma might necessitate a cerebrospinal fluid leak that has to be treated with a lumbar drain. Even more dramatically, when resecting intramedullary spinal tumors, vascular malformations or eloquent (i.e., growing close to or infiltrating functionally relevant brain areas) cerebral gliomas, there often is a very fine line between temporary and permanent surgery-related neurologic deficits that may have to be pushed to the limit to achieve the best possible outcome. Unavoidable temporary postoperative deficits and even a high likelihood of permanent postoperative disability can be acceptable for the patient and should be acceptable for the surgeon if this means a chance for longer survival or even cure for the patient.
The existence of expectable AEs that are intrinsic to specific treatments and deliberately accepted by the patient and the surgeon should be taken into account in future health care systems that might reimburse based on outcome.
Overall, the incidence of expectable AEs was 7.2% of all cases with AEs in cranial neurosurgery and 2.0% of all cases with AEs in spinal surgery.
4.3. How many adverse events can be avoided?
The vast majority of AEs is thus unexpected a priori. Unfortunately, many of them are also unavoidable in the sense that there is nothing that one would have done differently (e.g., a postoperative bleeding in the resection cavity leading to revision surgery that occurred even though there was meticulous hemostasis during the procedure and there were no signs of a coagulopathy beforehand; or a wound infection even though there were no avoidable patient-related or surgery-related risk factors, the wound closure was flawless and state-of-the-art hygiene protocols were followed during and after surgery).
However, in about one out of four cases with AEs, the AE was related to human error and thus potentially avoidable. This corresponds to 6.2% of all cranial neurosurgery patients and 6.7% of all spinal surgery patients. We found that most of these human performance deficiencies were execution errors (70.7%) or planning/problem solving errors (21.5%). A significant proportion especially of the execution errors may be related to the fact that our department has a neurosurgical training program. It is well-known that many neurosurgical procedures have a learning curve (Nowitzke, 2005; Ryang et al., 2015; Koc et al., 2006). Indeed, many execution errors were “hardware malposition” errors that occurred during procedures that are often performed by junior surgeons (e.g., a misplaced external ventricular device in cranial neurosurgery, or a misplaced pedicle screw in spinal surgery). It is conceivable that a surgical department that does not train residents could achieve lower rates of execution errors. However, training of future surgeons is a key duty and responsibility of any academic tertiary care center in our system, meaning that many of these AEs may actually also be hard to avoid. Specific training measures, such as a mandatory joint review of the operative result (e.g., pedicle screw positions) by the senior and junior surgeon, might be able to steepen learning curves for individual procedures, but this has to be evaluated prospectively in future studies.
4.4. Comparison with previous studies
There is no comparable study that prospectively investigated all AEs and the contribution of human error to AEs in cranial neurosurgery and spinal surgery inpatients. While previous reports have relied on retrospective reviews and/or focused on procedure-related or diagnosis-related complications (Schipmann et al., 2019; Wong et al., 2012a, 2012b, 2012c, 2012d; Houkin et al., 2009), we included all our inpatients and actively screened for any adverse event that occurred. Moreover, we determined whether human error contributed to an adverse event. Suliburk and colleagues presented a similar study based on a database of all surgeries performed by their general surgery, acute care surgery, surgical oncology, cardiothoracic surgery, vascular surgery and abdominal transplantation surgery services that are presented at weekly morbidity and mortality conferences (Suliburk et al., 2019). They report that AEs occurred in 182 out of 5365 patients undergoing surgery (3.4%) and that six AEs occurred in an unspecified number of patients undergoing non-operative treatment. As mentioned above, the overall AE rate in our study is much higher (25.0% of all patients had at least one AE). We cannot exclude that cranial neurosurgery and spinal surgery are more prone to AEs than other surgical disciplines at major academic medical centers. However, it seems likely that the low threshold we set for classifying an event as adverse, our active screening for AEs not only related to surgeries but also for AEs occurring on the ward, and our follow-up of all patients until discharge substantially contribute to this difference. This might also explain why the rate of human error - related AEs is lower in our study (25.9% vs. 56.4%), as AEs related to human performance deficiencies might be more noticeable than others and less likely to be omitted in mortality and morbidity conferences. Interestingly, the frequency of the different types of HPDs that Suliburk and colleagues introduced is comparable in our studies: We also found that the most frequent types are execution and planning/problem solving errors. This underlines the importance of these mostly cognitive errors and suggests that simulation-based cognitive training of surgeons in addition to systems-based safety measures, such as checklists and standard operating procedures, could provide a way to prevent avoidable AEs in cranial neurosurgery and spinal surgery.
5. Conclusion
Prospective data on the incidence of all types of AEs in neurosurgery bears significance not only for the education of our patients, but also for the discussion of quality-based accreditation and reimbursement systems in upcoming health care reforms.
This study showed that one in four patients treated at an academic neurosurgical tertiary care center experiences at least one adverse event. Of note, the high frequency of human error contributing to AEs in cranial neurosurgery and spinal surgery shows that there is potential to further eliminate avoidable patient harm.
Funding
This research did not receive any specific grant form funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Some of the results of this study have been presented at the 72nd Annual Meeting of the German Society of Neurosurgery (virtual plenary session, June 9th, 2021) and at the 15th Annual Meeting of the German Spine Society (virtual plenary session, December 11th, 2020).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bas.2021.100853.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Cohen M.E., Liu Y., Ko C.Y., Hall B.L. Improved surgical outcomes for ACS NSQIP hospitals over time: evaluation of hospital cohorts with up to 8 Years of participation. Ann. Surg. 2016;263(2):267–273. doi: 10.1097/SLA.0000000000001192. [DOI] [PubMed] [Google Scholar]
- Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawande A.A., Thomas E.J., Zinner M.J., Brennan T.A. The incidence and nature of surgical adverse events in Colorado and Utah in 1992. Surgery. 1999;126(1):66–75. doi: 10.1067/msy.1999.98664. [DOI] [PubMed] [Google Scholar]
- Gentry S., Badrinath P. Defining health in the Era of value-based care: lessons from England of relevance to other health systems. Cureus. 2017;9(3):e1079. doi: 10.7759/cureus.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S.J., Rolston J.D., Lau C.Y., Berger M.S. Improving patient safety in neurologic surgery. Neurosurg. Clin. 2015;26(2):143–147. doi: 10.1016/j.nec.2014.11.007. vii. [DOI] [PubMed] [Google Scholar]
- Hickey E.J., Halvorsen F., Laussen P.C., Hirst G., Schwartz S., Van Arsdell G.S. Chasing the 6-sigma: drawing lessons from the cockpit culture. J. Thorac. Cardiovasc. Surg. 2018;155(2):690–696. doi: 10.1016/j.jtcvs.2017.09.097. e691. [DOI] [PubMed] [Google Scholar]
- Houkin K., Baba T., Minamida Y., Nonaka T., Koyanagi I., Iiboshi S. Quantitative analysis of adverse events in neurosurgery. Neurosurgery. 2009;65(3):587–594. doi: 10.1227/01.NEU.0000350860.59902.68. discussion 594. [DOI] [PubMed] [Google Scholar]
- Koc K., Anik I., Ozdamar D., Cabuk B., Keskin G., Ceylan S. The learning curve in endoscopic pituitary surgery and our experience. Neurosurg. Rev. 2006;29(4):298–305. doi: 10.1007/s10143-006-0033-9. discussion 305. [DOI] [PubMed] [Google Scholar]
- Lawson E.H., Hall B.L., Louie R., et al. Association between occurrence of a postoperative complication and readmission: implications for quality improvement and cost savings. Ann. Surg. 2013;258(1):10–18. doi: 10.1097/SLA.0b013e31828e3ac3. [DOI] [PubMed] [Google Scholar]
- Leape L.L. Error in medicine. JAMA. 1994;272(23):1851–1857. [PubMed] [Google Scholar]
- Lucas D.J., Haider A., Haut E., et al. Assessing readmission after general, vascular, and thoracic surgery using ACS-NSQIP. Ann. Surg. 2013;258(3):430–439. doi: 10.1097/SLA.0b013e3182a18fcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowitzke A.M. Assessment of the learning curve for lumbar microendoscopic discectomy. Neurosurgery. 2005;56(4):755–762. doi: 10.1227/01.neu.0000156470.79032.7b. discussion 755-762. [DOI] [PubMed] [Google Scholar]
- Osborne N.H., Nicholas L.H., Ryan A.M., Thumma J.R., Dimick J.B. Association of hospital participation in a quality reporting program with surgical outcomes and expenditures for Medicare beneficiaries. JAMA. 2015;313(5):496–504. doi: 10.1001/jama.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter M.E., Lee T.H. The strategy that will fix health care. Harv. Bus. Rev. 2013;91(10):50–70. [Google Scholar]
- Rampersaud Y.R., Anderson P.A., Dimar J.R., 2nd, Fisher C.G., Spine Trauma Study G., Degenerative Spine Study G. Spinal Adverse Events Severity System, version 2 (SAVES-V2): inter- and intraobserver reliability assessment. J. Neurosurg. Spine. 2016;25(2):256–263. doi: 10.3171/2016.1.SPINE14808. [DOI] [PubMed] [Google Scholar]
- Rolston J.D., Bernstein M. Errors in neurosurgery. Neurosurg. Clin. 2015;26(2):149–155. doi: 10.1016/j.nec.2014.11.011. vii. [DOI] [PubMed] [Google Scholar]
- Ryang Y.M., Villard J., Obermuller T., et al. Learning curve of 3D fluoroscopy image-guided pedicle screw placement in the thoracolumbar spine. Spine J. : official journal of the North American Spine Society. 2015;15(3):467–476. doi: 10.1016/j.spinee.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Schipmann S., Brix T., Varghese J., et al. Adverse events in brain tumor surgery: incidence, type, and impact on current quality metrics. Acta Neurochir. 2019;161(2):287–306. doi: 10.1007/s00701-018-03790-4. [DOI] [PubMed] [Google Scholar]
- Suliburk J.W., Buck Q.M., Pirko C.J., et al. Analysis of human performance deficiencies associated with surgical adverse events. JAMA Netw. Open. 2019;2(7) doi: 10.1001/jamanetworkopen.2019.8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent C., Neale G., Woloshynowych M. Adverse events in British hospitals: preliminary retrospective record review. BMJ. 2001;322(7285):517–519. doi: 10.1136/bmj.322.7285.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman M., Takala R., Rahi M., Ikonen T.S. The need for surgical safety checklists in neurosurgery now and in the future-A systematic review. World neurosurgery. 2020;134:614–628 e613. doi: 10.1016/j.wneu.2019.09.140. [DOI] [PubMed] [Google Scholar]
- Wong J.M., Panchmatia J.R., Ziewacz J.E., et al. Patterns in neurosurgical adverse events: intracranial neoplasm surgery. Neurosurg. Focus. 2012;33(5):E16. doi: 10.3171/2012.7.FOCUS12183. [DOI] [PubMed] [Google Scholar]
- Wong J.M., Ziewacz J.E., Ho A.L., et al. Patterns in neurosurgical adverse events: cerebrospinal fluid shunt surgery. Neurosurg. Focus. 2012;33(5):E13. doi: 10.3171/2012.7.FOCUS12179. [DOI] [PubMed] [Google Scholar]
- Wong J.M., Ziewacz J.E., Ho A.L., et al. Patterns in neurosurgical adverse events: open cerebrovascular neurosurgery. Neurosurg. Focus. 2012;33(5):E15. doi: 10.3171/2012.7.FOCUS12181. [DOI] [PubMed] [Google Scholar]
- Wong J.M., Ziewacz J.E., Panchmatia J.R., et al. Patterns in neurosurgical adverse events: endovascular neurosurgery. Neurosurg. Focus. 2012;33(5):E14. doi: 10.3171/2012.7.FOCUS12180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.