Figure 1. Example of skeletal muscle relaxant object + co-dispensed drug of the base-pair episode eligible for inclusion.
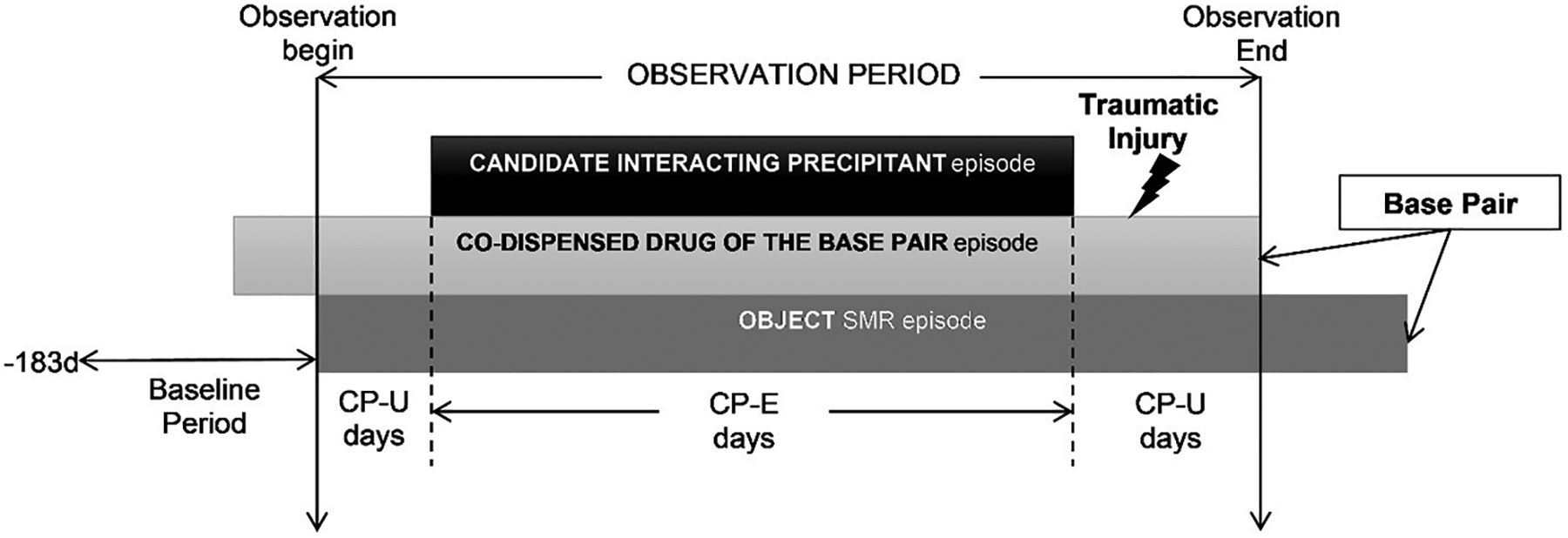
CP-E=candidate interacting precipitant-exposed; CP-U=candidate interacting precipitant-unexposed; SMR=skeletal muscle relaxant.
For each eligible SMR initiator (defined as having a ≥183-day washout period without SMR use before the first SMR prescription dispensing), the observation period began on the first day with supplies of both the SMR and the co-dispensed drug of the base-pair. The observation period consisted exclusively of days with continuous base-pair use. Days were categorized as candidate interacting precipitant exposed (CP-E) or unexposed (CP-U) based on whether the study individual had or did not have supply of a candidate interacting precipitant. The study individual was also required to experience a traumatic injury during the observation period. The comparison of interest was the rate of injury during CP-E days versus CP-U days.
