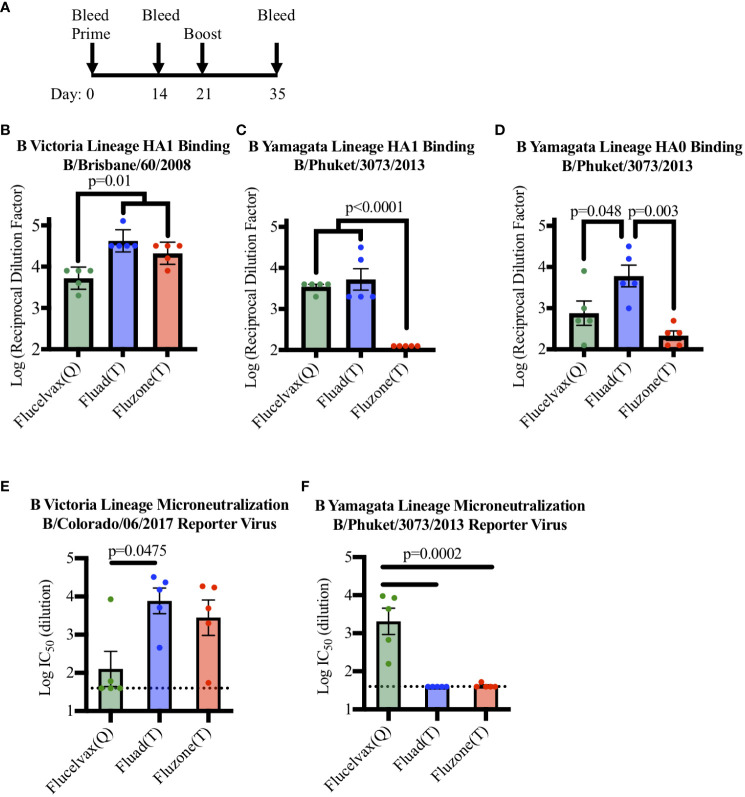
Figure 2.
Comparing antibody and microneutralization levels of vaccine sera against influenza B Victoria and influenza B Yamagata lineages: (A) Immunization schedule indicating days for the prime and boost for the vaccines (day 0 and 21) and bleeds (day 0, 14, and 35). (B–D) ELISA binding of immunized mouse sera to influenza B virus recombinant HA proteins: (B) HA1 of B-V (B/Brisbane/60/08), (C) HA1 of B-Y (B/Phuket/3073/13), (D) HA0 of B-Y (B/Phuket/3073/13), P-values are indicated for the significance of differences. (E, F) Testing microneutralization activities of vaccine sera using influenza B reporter viruses derived from (E) B-V (B/Colorado/06/2017) and (F) B-Y (B/Phuket/3073/2013). Vaccines were Flucelvax (Q) (green), Fluad (T) (blue) and Fluzone (T) (red). The dotted horizontal lines in panels E and F indicates the threshold for detection above background in the assay.