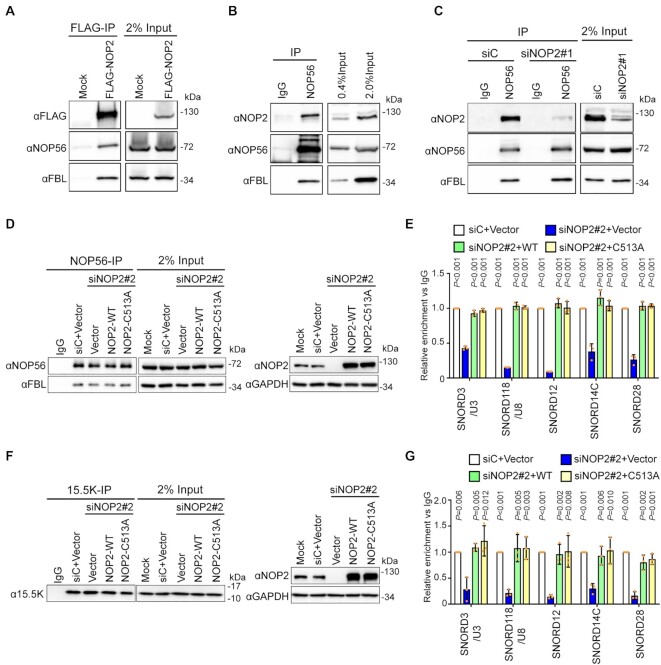
Figure 7.
NOP2/NSUN1 is required to maintain the integrity of C/D box snoRNPs. (A) HEK293T expressing empty vector (Mock) or FLAG-tagged NOP2/NUSN1 WT were lysed and immunoprecipitated (IP) with an anti-FLAG antibody. Associated proteins were analyzed by Western blot with the indicated antibodies. Mock transfected cells were used as negative control. (B) NOP56 was immunoprecipitated with an anti-NOP56 antibody from HEK293T cell lysate. Associated proteins were analyzed by Western blot with the indicated antibodies. Normal rabbit IgG was used as a negative immunoprecipitation control. (C) HEK293T cells were transfected with non-targeting control (siC) or NOP2 siRNA. 72 h later, cells were lysed and immunoprecipitated (IP) with an anti-NOP56 antibody. Associated proteins were analyzed by Western blot with the indicated antibodies. Normal rabbit IgG was used as a negative immunoprecipitation control. (D–G) HCT116 cells expressing empty vector (Vector), siRNA resistant NOP2/NSUN1 WT or the C513A catalytically inactive mutant were transfected with non-targeting control (siC) or NOP2 siRNA #2. After 72 h, cells were lysed and immunoprecipitated (IP) with anti-NOP56 (D, E) or anti-15.5K (F, G) antibody. A fraction of associated proteins was analyzed by western blot to control for immunoprecipitation and knockdown efficiency (D, F). The remaining immunoprecipitation fraction was processed for RNA extraction followed by RT-qPCR using SNORD3/U3, SNORD118/U8, SNORD12, SNORD14C, and SNORD28 specific primers (E, G). Relative enrichment over IgG control is represented. Data are presented as mean of three independent biological replicates ± standard deviation (SD). Statistical significance values relative to empty vector NOP2/NSUN1 knockdown (siNOP2 + Vector) were calculated using a two-tailed independent Student's t-test.