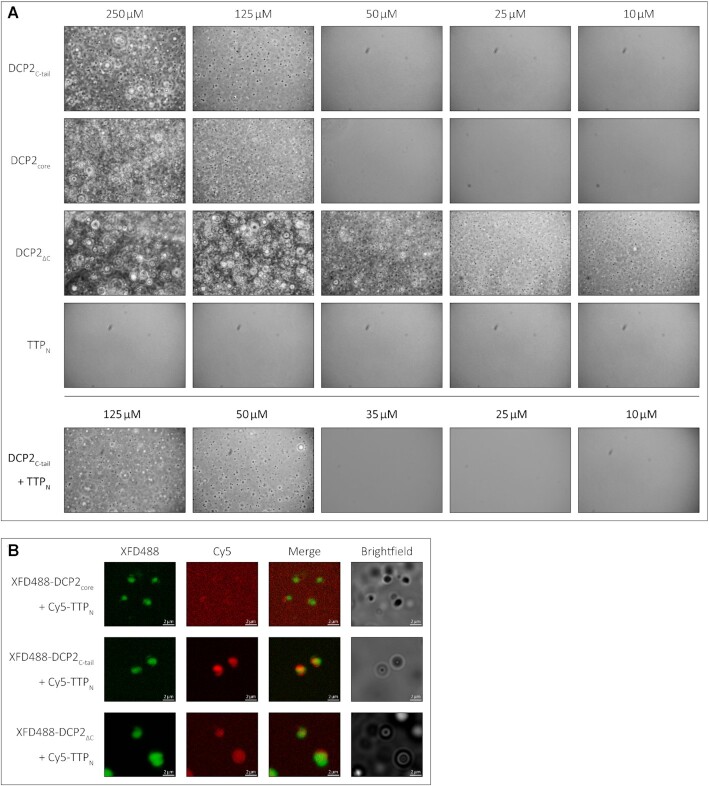
Figure 4.
The low-affinity interaction of TTP with DCP2 induces LLPS of TTPNin vitro. (A) Representative images of in vitro LLPS of the DCP2 proteins and TTPN in the presence of a molecular crowding reagent, PEG8000. A range of protein concentrations from 250 to 10 μM were analysed. All DCP2 proteins undergo LLPS at high concentrations, while DCP2ΔC, which has the strongest tendency to undergo phase separation, also does so at the lowest concentration investigated. TTPN does not form phase-separated droplets in any of the tested conditions. Mixtures of TTPN and DCP2C-tail show phase separation at a lower concentration than DCP2C-tail alone. Images were acquired by phase-contrast microscopy. (B) Representative images of in vitro phase separation of equimolar (90 μM) mixtures of Cy5-labelled TTPN and XFD488-labelled DCP2 in the presence of a molecular crowding reagent, PEG8000. Overlap of Cy5 and XFD488 signals suggests co-localization of the two proteins. TTPN partitions into phase-separated droplets in the presence of DCP2C-tail and DCP2ΔC, but not in the presence of DCP2core. Brightfield images show additional phase-separated droplets in different focal planes that are not visible in the fluorescent images due to optical sectioning by the Zeiss Apotome. The imperfect overlap between the XFD488 and Cy5 fluorescent images stems from moving droplets and a time lag in image acquisition. Scale bars, 2 μm.