Figure 2.
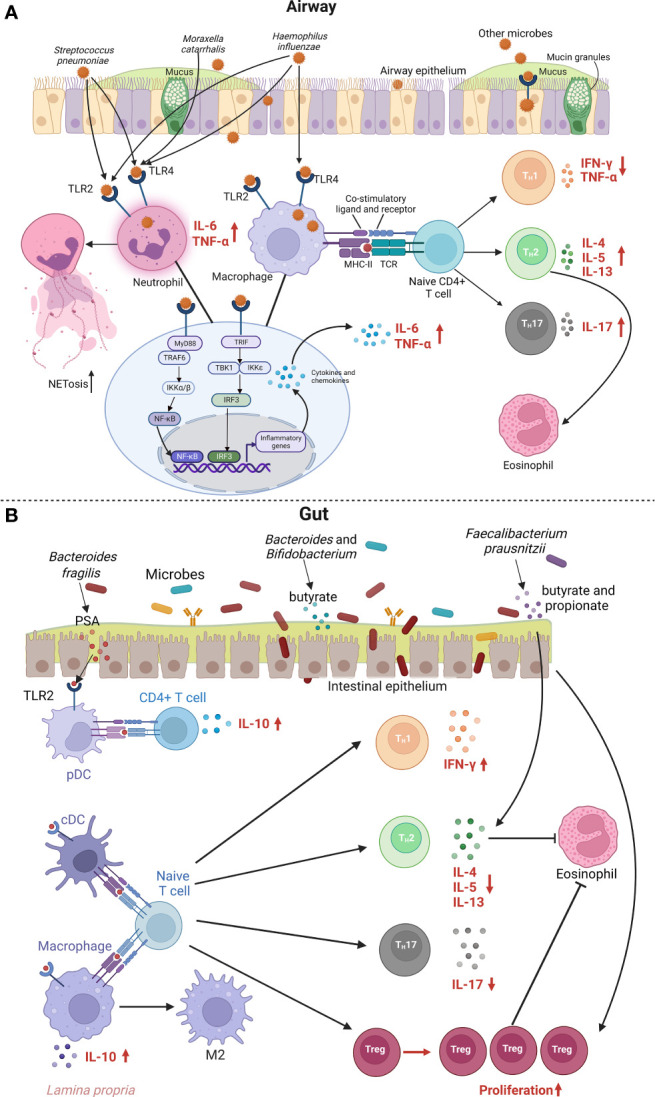
Metabolic and immune mechanisms for the link between airway or gut microbiota and asthma. (A) shows immune mechanisms for the airway epithelial cell signaling in response to bacterial pathogens. Various bacterial pathogens bind to innate sensors TLR2 and TLR4 and further activate MyD88- and TRIF-dependent pathways. MyD88 recruits TRAF6, which further activates the IKK complex, allows NF-кB to translocate into the nucleus, and leads to the overall production of inflammatory cytokines and chemokines, and activation of T cells. Additionally, recognition of various bacterial pathogens activates the TRIF-dependent signaling pathway, which involves the recruitment of TRIF, that leads to subsequent activation of TBK1 and IKKϵ along with induction of transcription factor IRF3. This signaling pathway results in interferon-related cytokines, and can potentiate NF-κB gene transcription. Enhanced neutrophil TLR2 and TLR4 signaling by bacterial pathogens promote neutrophils cytokine production and NETosis, a program for formation of NETs. Bacterial pathogens can also be recognized by TLR2 and TLR4 on macrophages, leading to activation of NF-κB and IRF3 signaling pathway and secretion of inflammatory mediators. Macrophages can also function as APC and regulate T cell activation. The T cell is presented an antigen with MHC II by APC. The recognition of the antigen-MHC II complex and the co-stimulatory molecules activates the T cell and leads downstream to differentiation into TH2 and TH17 cells, that can release various cytokines such as IL-4, IL-5 and IL-13, which lead to eosinophilic inflammation. APC, antigen-presenting cell; IKK, inhibitory kappa B kinases; IL, interleukin; IRF3, interferon regulatory factor 3; MHC, major histocompatibility complex; MyD88, myeloid differentiation primary response protein 88; NETosis, neutrophil extracellular trap formation; NETs, neutrophil extracellular traps; NF-кB, nuclear factor kappa B; TBK1, TANK-binding kinase 1; TH2, T helper 2; TH17, T helper 17; TLR, Toll-like receptor; TRAF6, tumor necrosis factor receptor associated factor 6; TRIF, TIR-domain-containing adapter-inducing interferon-β. (B) shows metabolic and immune mechanisms for the link between gut microbiota and asthma. PSA from Bacteroides fragilis induces and ligates TLR2 on pDC, which stimulate anti-inflammatory cytokine IL-10 secretion by CD4+ T cells. cDC and macrophage bound by gut microbiota show impaired ability to promote TH2- and TH17-type responses and tend to promote TH1-type responses and Treg proliferation, which lead to decreased eosinophilic inflammation. IL-10 dependent reprogramming of tissue macrophages is also essential for resolving inflammation by promoting M2 macrophage polarization. Bacteroides and Bifidobacterium can digest the fiber and produce SCFAs, such as butyrate. Faecalibacterium prausnitzii increases SCFAs level, such as butyrate and propionate, which leads to reduced levels of IL-4, IL-5 and IL-13, and elevated level of Tregs. cDC, classical dendritic cell; IFN, interferon; IL, interleukin; pDC, plasmacytoid dendritic cell; PSA, polysaccharide A; SCFAs, short-chain fatty acids; TH2, T helper 2; TH17, T helper 17; TLR, Toll-like receptor; Treg, regulatory T cell.
