Figure 4.
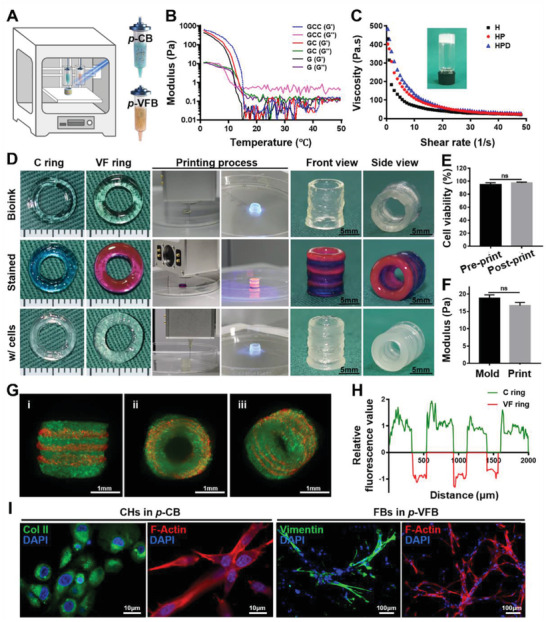
Printability evaluation of bioinks and characterizations of bioprinted CVFIT. A) Schematic of the dual‐nozzle 3D extrusion‐based bioprinting for CVFIT construction based on tissue‐specific bioinks of p‐CB and p‐VFB. B) Temperature‐sweep rheological analyses indicating the printability of p‐CB based on its temperature‐sensitive property. C) Viscosity profiles implying the printability of p‐VFB based on its relatively high viscosity and shear‐thinning properties. D) Photographs of the 3D‐printed trachea‐analogues, dye‐stained bioinks for visualization, and bioprinted CVFIT using cell‐loaded bioinks. E) Cell viability and F) mechanical properties of bioinks after molding or 3D printing. G) Lightsheet microscopy images for visualizing cell distributions in the bioprinted CVFIT. GFP‐labeled chondrocytes (green); RFP‐labeled fibroblasts (red). H) Relative fluorescence intensity analyses of the bioprinted CVFIT using ImageJ. I) Immunofluorescent staining of Col II for chondrocytes in p‐CB, and vimentin for fibroblasts in p‐VFB. CVFIT: cartilage‐vascularized fibrous tissue‐integrated trachea; p‐CB: photocrosslinkable cartilage‐specific bioink; p‐VFB: photocrosslinkable vascularized fibrous tissue‐specific bioink;C ring: cartilage ring; VF ring: vascularized fibrous tissue ring.
