Abstract
Objectives
: We aimed to investigate the 1-month humoral response to two or three doses of a messenger RNA coronavirus disease 2019 (COVID-19) vaccine as a primary vaccination regimen in specific populations compared with that in healthy adults.
Methods
Agence Nationale Recherche contre le Sida (ANRS)0001S–COV-POPART (NCT04824651) is a French nation-wide, multi-centre, prospective, observational cohort study assessing the immune response to COVID-19 vaccines routinely administered to 11 sub-groups of patients with chronic conditions and two control groups. Patients and controls who received at least two vaccine doses and whose results 1 month after the second dose were available were included. The humoral response was assessed 1 month after the first, second and third doses (if applicable) based on the percentage of responders (positive for anti-Spike severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] IgG antibodies), geometric means of anti-Spike SARS-CoV-2 IgG antibodies (enzyme-linked immunosorbent assay) and proportion of participants with anti-SARS-CoV-2-specific neutralizing antibodies (in vitro neutralization assay for the original SARS-CoV-2 strain). All analyses were centralized.
Results
We included 4091 participants in this analysis: 2979 participants from specific sub-populations and 1112 controls. Only 522 (17.5%) participants from the specific populations received three doses as a primary vaccination regimen. Patients living with human immunodeficiency virus, cancer and diabetes had high percentages of responders after two doses, whereas patients with solid organ transplants, allogeneic hematopoietic stem cell transplants and hypogammaglobulinaemia had the lowest percentage of responders (35.9% [95% CI, 29.2–43.0], 57.4% [95% CI, 48.1–66.3] and 77.1% [95% CI, 65.6–86.3], respectively). In those who received the third dose, the percentage of responders reached 54.2% (95% CI, 42.9–65.2) (vs. 32.3% [95% CI, 16.7–51.4] after 2 doses) among those with solid organ transplants and 73.9% (95% CI, 58.9–85.7) (vs. 56.1% [95% CI, 46.2–65.7] after 2 doses) among those with hematopoietic stem cell transplants. Similar results were found with anti-SARS-CoV-2-specific neutralizing antibodies.
Conclusions
A lower humoral response to COVID-19 vaccines was observed in the specific populations compared with that in the controls. The third dose of this vaccine in the primary regimen had a positive effect on the percentages of patients who developed anti-Spike IgG antibodies and specific neutralizing antibodies.
Keywords: COVID-19, Efficacy, Humoral, Immunocompromised, Immunogenicity, Specific populations
Introduction
Specific populations, such as those of immunocompromised patients or patients with obesity, are defined as individuals at the risk of developing severe forms of infectious diseases and in whom there are concerns of potentially decreased immunogenicity and effectiveness of vaccines. Phase 3 studies on coronavirus disease 2019 (COVID-19) vaccines have provided little information on vaccine efficacy in specific populations. Initial real-life studies showed lower anti-Spike antibody responses [1,2], leading to lower vaccine efficacy compared with those in non-immunocompromised populations [[3], [4], [5], [6]]. Most of these studies were based on small sample sizes, with no standardized measures and control groups [1,2]. Many countries have recommended at least one additional dose in the primary vaccination regimen in immunocompromised populations, with the results showing convincing effects on the humoral response in small studies [7].
Detailed short- and long-term immune response assessments in these populations based on the number of doses of the vaccine received are warranted to inform reflections on tailored vaccine regimens.
The ANRS0001S COV-POPART cohort study aimed at assessing the humoral immune responses to COVID-19 vaccines in 11 sub-groups of specific populations compared with those in a control group [8].
Methods
Study design and patients
ANRS0001S–COV-POPART (NCT04824651) is a multi-centre, prospective cohort study conducted in France to assess the immune response to COVID-19 vaccines routinely administered to specific populations as part of a national immunization campaign [8].
The study was conducted in 36 participating centres in France in collaboration with the I-reivac network, ten national learned societies and seven patients' associations (France Rein, Transhépate, Fondation pour l’Aide à la Recherche sur la Sclérose en Plaques (ARSEP Foundation), Collectif National des Associations d'Obèses (CNAO), Fédération Francaise des Diabétiques (FFD), Association d'Entraide aux Greffés de Moelle Osseuse (EGMOS) and Traitements et Recherche Thérapeutique - 5 Collectif Hépatites Virales (TRT5 CHV)).
Adults without a known history of COVID-19 and affected by solid cancer, solid organ transplant (SOT), allogeneic hematopoietic stem cell transplant (HSCT), chronic renal failure (stage 4 and 5 = glomerular filtration rate <30 mL/min/1.73 m2) with or without dialysis, multiple sclerosis (MS) or neuromyelitis optica spectrum disorders (NMOSDs), inflammatory rheumatic diseases, systemic autoimmune diseases, hypogammaglobulinemia (primary and secondary), obesity (body mass index >30 kg/m2) or diabetes mellitus (both types 1 and 2) or patients living with human immunodeficiency virus (PLWHIV) and willing to be vaccinated as part of the national immunization campaign were included from 25 March, 2021, to 31 December, 2021. Patients might have been included before any vaccination, after the first dose or in the month following the second dose. Patients could have been affected by more than one chronic condition and, thus, included in different specific populations. In this study, they have been described in each of their population of inclusion.
One control group without any of the above-mentioned underlying conditions was also included. This control group was initially stratified into two groups: age <75 and ≥ 75 years. However, the recruitment of patients aged ≥75 years was not successful. For statistical analyses, we considered participants aged <65 and ≥ 65 years.
The main exclusion criteria were pregnancy or breastfeeding, history of known severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, acute febrile infection within the previous 72 hours, symptoms suggestive of COVID-19 or contact with a case within the last 14 days prior to the inclusion visit, history of severe post-vaccination adverse events or severe allergic manifestations, and receipt of another vaccine within 4 weeks prior to the first injection or scheduled to receive a licensed vaccine in the 4 weeks following inclusion.
Patients and controls who had received at least two doses of a messenger RNA (mRNA) vaccine (i.e. patients who received one dose of the COVID-19 vaccine Janssen or a heterologous vaccination regimen, such as ChAdOx1-nCoV19/BNT162b2, were excluded) and whose results 1 month after the second dose were available were included. Furthermore, participants positive for SARS-CoV-2 anti-nucleocapsid antibodies before vaccination or at any follow-up visit were excluded.
The details of COVID-19 vaccination are available in the supplementary material.
Sample collection and laboratory assays
Serum samples were collected at inclusion, 1 month after dose 1, 1 month after dose 2 and 1 month after dose 3 (if applicable) for this analysis. Data collected between 21 and 56 days after the injection were considered as measured 1 month after the dose. The samples collected at each site were sent to a certified core laboratory (CRB Bordeaux) before being sent for serological analysis to the ‘Unité des virus émergents’ Aix-Marseille Université, Institut de Recherche pour le Développement 190, Inserm 1207, Institut Hospitalo-Universitaire Méditerranée Infection, Marseille, France.
The details of the laboratory assay are available in the supplementary material.
Outcomes
The main outcome measures were the percentage of responders (positive anti-Spike SARS-CoV-2 IgG antibodies (enzyme-linked immunosorbent assay); a ratio of optic density of the sample to that of a calibrator of ≥1.1, as defined by EuroImmun serology); geometric means titres of anti-Spike SARS-CoV-2 IgG antibodies, expressed in binding antibody units per millilitre (BAU/mL) and anti-SARS-CoV-2-specific neutralizing antibodies (in vitro neutralization assay for the original SARS-CoV-2 strain) 1 month (21–56 days) after the last dose of the primary vaccination regimen (two or three doses). Quantitative distributions were described only for responders' participants for this antibody. Responders were categorized as weak (anti-Spike SARS-CoV-2 IgG level <264 BAU/mL) [9], moderate (264–1350 BAU/mL) and strong (>1350 BAU/mL, corresponding to the median anti-Spike IgG level observed in all the controls).
The proportion of participants with sero-neutralization antibodies (a titre of ≥20) was described as percentages.
The details of statistics are available in the supplementary material.
Ethics
Written informed consent was obtained from each participant before enrolment taking into account the European Union General Data Protection Regulation requirements. The protocol (N° EudraCT/ID-RCB: 2021-A00348-33) was conducted in accordance with the Declaration of Helsinki and French law for research involving human subjects (known as Loi Jardé). The protocol was approved by the following ethics committees: the committee for the protection of persons engaged in research ‘CPP Nord-Ouest IV’ (file number: 21.02.12.47147) and the French national data protection authority Commission Nationale Informatique et Liberté (authorization number: 921111v1).
Results
Patients
Of 6108 participants recruited until 31 December, 2021, 4348 (71%) had results available 1 month after the second dose of the primary vaccination regimen at the time of the first analysis, of whom 4091 (67%) had no anti-nucleocapsid antibodies: 2979 participants from specific sub-populations and 1112 controls (Fig. S1). The details of the number of patients included in each sub-population are displayed in Table 1 . The details of the characteristics of the participants included in each sub-population are displayed in Tables S1–S11. The overall median age of the patients was 51.1 years (interquartile range, 40.3–60.4), and 2047 (50.0%) patients were men. Most participants received at least two doses of the BNT162b2 vaccine (3581, 87.5%). In the specific populations, 522 (17.5%) patients received 3 doses as the primary vaccination regimen, and most of them had HSCT (n = 107, 87.7% of this sub-population) or SOT (n = 167, 84.3% of this sub-population). The median time between the second and third doses was 4.1 weeks (interquartile range, 4.0–4.9 weeks). Patients with HSCT who received 3 doses were older (54.7 years [interquartile range, 39.5–65.6 years] vs 52.5 years [interquartile range, 41.0–56.5 years]) and more likely to have received the allograft <3 years prior (94.2% vs 60.0%) than those who received only two doses. Patients with SOT who received 3 doses were older (58.3 years [interquartile range, 48.0–66.4 years] vs 56.6 years [interquartile range, 47.8–65.6 years]), had received T-cell-depleting agents more recently and were more likely to be recipients of an organ (heart, lung, liver or pancreas) other than the kidney than those who received only two doses.
Table 1.
Characteristics of patients and controls included in the preliminary analyses of the ANRS0001 S COV-POPART cohort study
| Specific population (n = 2979) |
Control group <65 y (n = 962) |
Control group ≥65 y (n = 150) |
||||
|---|---|---|---|---|---|---|
| Characteristics | n | Median (IQR) or n (%) | n | Median (IQR) or n (%) | n | Median (IQR) or n (%) |
| Age (y) | 2974 | 52.5 (41.9–61.3) | 962 | 43.9 (34.1–52.1) | 150 | 70.8 (68.1–74.7) |
| Men | 2979 | 1504 (50.5) | 962 | 473 (49.2) | 150 | 70 (46.7) |
| Population | 2979 | — | — | |||
| Cancer | 188 (6.3) | — | — | |||
| Solid organ transplant | 198 (6.6) | — | — | |||
| Hematopoietic stem cell transplant | 122 (4.1) | — | — | |||
| Chronic renal failure with or without dialysis | 95 (3.2) | — | — | |||
| Systemic autoimmune diseases | 173 (5.8) | — | — | |||
| Inflammatory rheumatic diseases | 191 (6.4) | — | — | |||
| Multiple sclerosis or neuromyelitis optica spectrum disorders | 416 (14.0) | — | — | |||
| Hypogammaglobulinemia | 70 (2.3) | — | — | |||
| Diabetes | 445 (14.9) | — | — | |||
| Non-diabetic with obesity | 820 (27.5) | — | — | |||
| Patients with HIV | 897 (30.1) | — | — | |||
| Type of vaccine | 2919 | 955 | 149 | |||
| BNT162b2 + BNT162b2 | 2163 (74.1) | 854 (89.4) | 138 (93.3) | |||
| BNT162b2 + BNT162b2 + BNT162b2 | 426 (14.6) | — | — | |||
| mRNA-1273 + mRNA-1273 | 230 (7.9) | 92(9.6) | 10 (6.7) | |||
| mRNA-1273 + mRNA-1273 + mRNA-1273 | 78 (2.7) | — | — | |||
| BNT162b2 + mRNA-1273 | 15 (0.5) | 9 (0.9) | 0 (0.0) | |||
| BNT162b2 + BNT162b2 + mRNA-1273 | 3 (0.1) | — | — | |||
| BNT162b2 + mRNA-1273 + mRNA-1273 | 4 (0.1) | — | — | |||
| Number of doses | 2979 | 962 | 150 | |||
| 2 | 2457 (82.5) | 962 (100.0) | 150 (100.0) | |||
| 3 | 522 (17.5) | 0 (0.0) | 0 (0.0) | |||
| Hemoglobin (g/dL) | 2296 | 14.1 (13.0–15.2) | 872 | 14.0 (13.2–15.1) | 146 | 14.0 (13.4–14.8) |
| White blood cells (cells/μL) | 2294 | 6390 (5130–7800) | 871 | 6000 (5130–7100) | 145 | 6180 (5300–7130) |
| Neutrophils (cells/μL) | 2284 | 3700 (2800–4750] | 870 | 3480 (2890–4320) | 145 | 3770 (2920–4570) |
| Platelets (10/mm3) | 2292 | 249 (210–292) | 869 | 250 (215–284) | 146 | 247 (219–280) |
| Creatinine (μmol/L) | 2276 | 72.0 (62.0–87.0) | 867 | 70.0 (61.0–80.0) | 144 | 71.0 (64.0–81.5) |
| Alanine aminotransferase (IU/L) | 730 | 25.0 (18.0–37.0) | 0 | — | 0 | — |
| Aspartate aminotransferase (IU/L) | 729 | 22.0 (18.0–28.5) | 0 | — | 0 | — |
| C-reactive protein (mg/L) | 2070 | 3.0 (1.1–4.6) | 787 | 1.2 (1.0–4.0) | 100 | 3.8 (1.0–4.0) |
| C-reactive protein ≥5 mg/L | 2070 | 416 (19.6) | 787 | 37 (4.7) | 100 | 9 (9.0) |
| Lymphocytes <1000 G/L | 2284 | 225 (9.6) | 870 | 44 (5.1) | 145 | 7 (4.8) |
All characteristics were recorded at the time of inclusion into the cohort. HIV, human immunodeficiency virus; IQR, interquartile range.
One-month humoral response following two doses of COVID-19 mRNA vaccines
Percentage of responders
In the control group, 100% (95% CI, 99.6–100.0) of those aged 18–64 years and 99.3% (95% CI, 96.3–100.0) of those aged >65 years developed anti-Spike IgG antibodies.
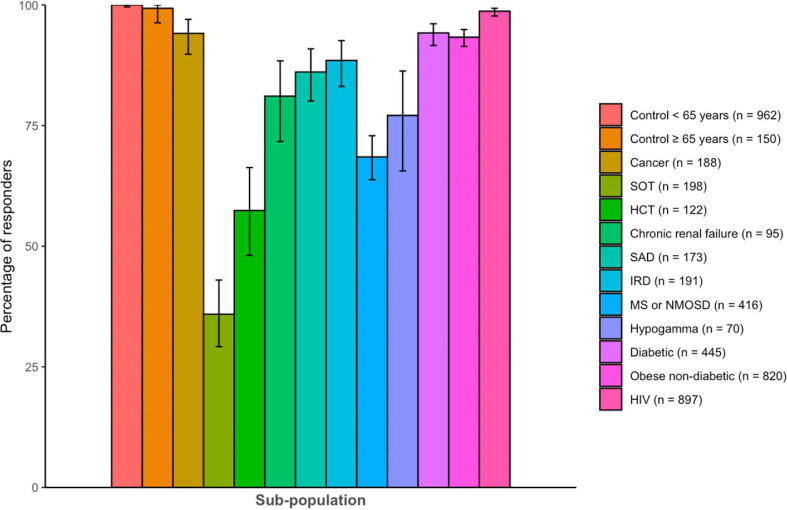
PLWHIV and patients with diabetes, cancer and obesity had high percentages of responders, at 98.7% (95% CI, 97.7–99.3), 94.2% (95% CI, 91.6–96.2), 94.2% (95% CI, 89.8–97.0) and 93.3% (95% CI, 91.4–94.9), respectively. The lowest percentage of responders was found in populations with SOT (35.9% [95% CI, 29.2–43.0]), HSCT (57.4% [95% CI, 48.1–66.3]), MS or NMOSD (68.5% [95% CI, 63.8–73.0]) and hypogammaglobulinaemia (77.1% [95% CI, 65.6–86.3]) (Fig. 1 and Table S12). Patients with chronic renal failure, systemic autoimmune diseases, inflammatory rheumatic diseases and MS or NMOSD had intermediate responses, although lower than those of the control populations. Compared with the control participants, the response was significantly lower in each population (p <0.01 in each population). After adjustment for age and sex, the difference was still significant (p <0.01 in each population, except for participants with human immunodeficiency virus, p 0.01).
Fig. 1.
Percentage of responders with anti-Spike antibodies 1 month after the second dose of anti-severe acute respiratory syndrome coronavirus 2 messenger RNA vaccine in the different sub-populations and controls of the ANRS0001 S COV-POPART cohort. The vertical bars indicate a 95% CI. HCT, hematopoietic stem cell transplant; HIV, human immunodeficiency virus; IRD, inflammatory rheumatic disease; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorder; SAD, systemic autoimmune disease; SOT, solid organs transplanted.
Strength of the anti-Spike IgG antibody response
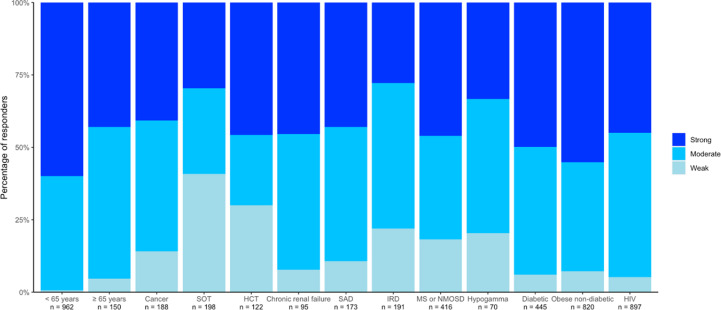
In the control group, 0.6% (95% CI, 0.2–1.4) of those aged 18–64 years and 4.7% (95% CI, 1.9–9.4) of those aged >65 years were categorized as weak responders. The percentage of weak responders was higher in all the sub-populations than in the controls, with the highest percentages found in patients with SOT (40.9% [95% CI, 29.3–53.2]) and HSCT (30.0% [95% CI, 19.6–42.1]). The percentage of strong responders was highest in controls aged <65 years (59.9% [95% CI, 56.7–63.0]) and lowest in patients with SOT (29.6% [95% CI, 19.3–41.6]) and hypogammaglobulinaemia (33.3% [95% CI, 21.1–47.5]). Interestingly, some sub-populations had higher percentages of strong responders than controls aged >65 years (Fig. 2 and Table S13).
Fig. 2.
Distribution of responders according to the strength of anti-Spike antibodies response at 1 month after the second dose of an anti-severe acute respiratory syndrome coronavirus 2 vaccine in the different sub-populations and controls of the ANRS0001 S COV-POPART cohort. HCT, hematopoietic stem cell transplant; HIV, human immunodeficiency virus; IRD, inflammatory rheumatic disease; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorder; SAD, systemic autoimmune disease; SOT, solid organs transplanted.
Anti-SARS-CoV-2-specific neutralizing antibodies
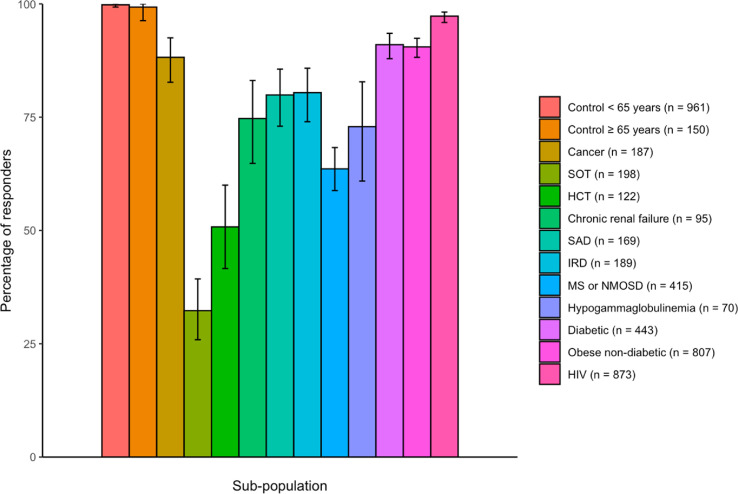
In the control group, 99.8% (95% CI, 99.3–100.0) of those aged 18–64 years and 99.3% (95% CI, 96.3–100.0) of those aged >65 years had specific neutralizing antibodies against the SARS-CoV-2 D614 strain. PLWHIV, patients with diabetes and obesity and non-diabetic patients had the highest percentages of patients with neutralizing antibodies, at 97.3% (95% CI, 95.9–98.2), 91.0% (95% CI, 87.9–93.5) and 90.5% (95% CI, 88.2–92.4), respectively. The lowest percentages of responders were among patients with SOT (32.3% [95% CI, 25.9–39.3]), HSCT (50.8% [95% CI, 41.6–60.0]) and hypogammaglobulinaemia (72.9% [95% CI, 60.9–82.8]) (Fig. 3 and Table S14). Again, patients with chronic renal failure, systemic autoimmune diseases, inflammatory rheumatic diseases and MS or NMOSD had intermediate responses, although lower than those of the control populations. The response in each population was significantly lower than that in the control participants before and after adjustment for age and sex (both p <0.01 in each population).
Fig. 3.
Percentage of responders with neutralizing antibodies 1 month after the second dose of an anti-severe acute respiratory syndrome coronavirus 2 vaccine in the different sub-populations and controls of the ANRS0001 S COV-POPART cohort. The vertical bars indicate 95% CI. HCT, hematopoietic stem cell transplant; HIV, human immunodeficiency virus; IRD, inflammatory rheumatic disease; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorder; SAD, systemic autoimmune disease; SOT, solid organs transplanted.
One-month humoral response following three doses of COVID-19 mRNA vaccines
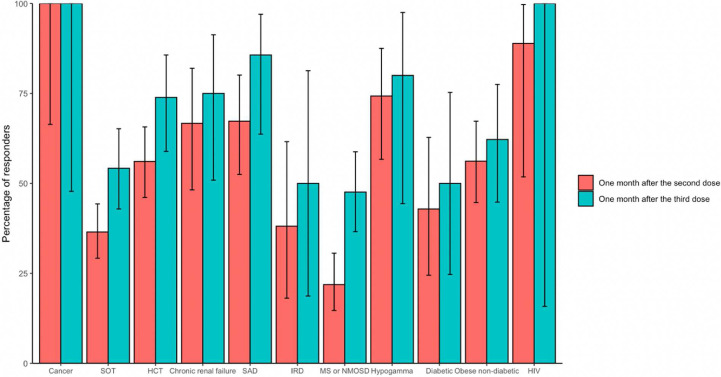
The third dose added as primary vaccination increased the percentage of responders among those who received a third dose in all concerned sub-populations, reaching 54.2% (45/83) (95% CI, 42.9–65.2) (vs 36.5 [95% CI, 29.2–44.3] after dose 2) in patients with SOT and 73.9% (34/46) (95% CI, 58.8–85.7) (vs 56.1% [95% CI, 46.2–65.7] after dose 2) in patients with HSCT (Fig. 4 and Table S15). Of note, the geometric mean titres of anti-Spike SARS-CoV-2 IgG antibodies after three doses reached levels similar to those after two doses in those who received only two doses, except in patients with MS or NMOSD and hypogammaglobulinaemia (Fig. S2 and Table S16).
Fig. 4.
Percentage of responders with anti-Spike antibodies 1 month after the second and 1 month after the third dose of an anti-severe acute respiratory syndrome coronavirus 2 vaccine in participants from the ANRS0001S COV-POPART cohort who received three doses as a primary vaccination regimen. The vertical bars indicate 95% CI. HCT, hematopoietic stem cell transplant; HIV, human immunodeficiency virus; IRD, inflammatory rheumatic disease; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorder; SAD, systemic autoimmune disease; SOT, solid organs transplanted.
The percentage of patients with neutralizing antibodies increased in all the sub-populations, reaching 47.0% (95% CI, 35.9–58.3) (vs 32.9% [95% CI, 25.9–40.6]) after dose 2, p 0.01) in patients with SOT and 69.8% [95% CI, 53.9–82.8] (vs 48.6% [95% CI, 38.8–58.5] after dose 2, p 0.05) in patients with HSCT. The increase was only moderate in patients with MS or NMOSD (30.4% [95% CI, 20.5–41.8) vs 16.7% [95% CI, 10.3–24.8] after dose 2) and inflammatory rheumatic diseases (40.0% [95% CI, 12.2–73.8] vs 23.8% [95% CI, 8.2–47.2] after dose 2) (Fig. S3 and Table S17). The geometric mean titres of neutralizing antibodies reached levels similar to the ones 1 month after dose 2 in those who received only two doses (Fig. S4 and Table S18).
Anti-cluster of differentiation (CD)20 treatment as part of the immuno-suppressive treatment regimen in patients with MS or NMOSD and autoimmune inflammatory rheumatic diseases was frequent (32.0% and 11.0% in the study, respectively). The percentage of responders among patients with MS or NMOSD receiving anti-CD20 who received a third dose reached 40.9% (95% CI, 29.0–53.7) (vs 16.7% [95% CI, 9.6–26.0] after dose 2) compared with 72.2% (95% CI, 46.5–90.3) (vs 41.7% [95% CI, 22.1-63.4] after dose 2) in those with MS or NMOSD who did not receive anti-CD20 and received a third dose.
Overall humoral response 1 month after the end of the primary COVID-19 mRNA vaccine regimen (two or three doses)
When the humoral responses 1 month after the second dose in those who received only two doses and were pooled with those 1 month after the third dose in those who received three doses, we observed that the control groups and all the specific population groups, except patients with SOT, HSCT, chronic kidney disease, MS or NMOSD and hypogammaglobulinaemia, had ≥90% responders with both anti-Spike SARS-CoV-2 IgG and neutralizing antibodies. The lowest percentage of responders was among patients with SOT, with 48.3% (95% CI, 38.8–57.8) and 42.1% (95% CI, 32.9–51.7) for anti-Spike SARS-CoV-2 IgG and neutralizing antibodies, respectively (Table S19). The overall proportion of strong responders was 50.5% (95% CI, 48.8–52.1) among sero-responders, 47.3% (95% CI, 45.3–49.3) in specific populations and 59.9% (95% CI, 56.7–63.0) in controls aged <65 years. Patients with autoimmune inflammatory rheumatic diseases had the lowest percentage of high responders, followed by those with SOT, despite a high proportion of patients who received three doses as the primary vaccination regimen in the latter, and hypogammaglobulinaemia (Table S20).
Discussion
The ANRS0001S COV-POPART cohort is, to the best of our knowledge, the largest cohort study that has assessed the response to COVID-19 vaccines in specific populations to date.
These results showed that, first, the humoral response in these populations 1 month after receiving two doses of mRNA COVID-19 vaccines was heterogeneous compared with that in the controls. This response was noticeably low in patients with SOT, HSCT, hypogammaglobulinemia and MS or NMOSD. On the contrary, PLWHIV and patients with obesity, cancer or diabetes had good response rates. Second, the strength of the humoral neutralizing response was lower in all the sub-groups investigated in this study compared with that in the control group. Third, the third dose of the vaccine in the primary regimen had a positive effect on the humoral response 1 month after the third dose and on the percentages of patients who developed anti-Spike IgG antibodies and specific neutralizing antibodies. However, this effect was only moderate in some sub-groups, such as participants with MS or NMOSD, hypogammaglobulinaemia and inflammatory rheumatic diseases, because a lower increase was observed in the geometric mean titres of anti-Spike IgG antibodies and specific neutralizing antibodies compared with those in patients who received only two doses.
These results are in line with the existing literature showing overall blunted humoral responses in immunocompromised populations [1,2], which can be increased by a third [7] or fourth [10,11] additional dose of COVID-19 mRNA vaccine. We highlighted the negative impact of anti-CD20 treatment on the response to additional doses in patients with MS or NMOSD and inflammatory rheumatic diseases, as previously shown in the literature in patients who received two doses [12,13] or more [14]. This could also contribute to an increased risk of severe COVID-19 in patients with MS treated with anti-CD20 [15].
Although there is no clear correlation of protection, especially in the context of the circulation of Omicron variants, it is important to induce high antibody titres and cellular responses in populations at a high risk of severe COVID-19. Thus, the monitoring of anti-Spike IgG antibodies following vaccination is important to allow the indication of a third dose of the vaccine and the use of SARS-CoV-2 monoclonal antibodies as pre-exposure prophylaxis. The assessment of heterologous vaccine schemes, especially with adjuvanted recombinant protein vaccines, would be of interest, as would be the evaluation of the persistence of the humoral response.
Our study has some limitations, including the inherent bias of a cohort (i.e. descriptive data, possible lack of representativeness of the included participants in each sub-population), limited sample size for statistics in some populations, lack of cellular response data and lack of data on neutralizing antibodies against the Omicron variant of concern.
The strengths of this study include its large sample with several different specific populations and two control groups with geographical representativity of the country, standardized timeline of assessment, and standardized, centralized analyses of the results.
In conclusion, these initial results show that specific populations had a lower humoral response 1 month after receiving two doses of COVID-19 mRNA vaccines than the general population. This response was heterogeneous mong the sub-populations. Our results highlight the importance of the assessment of the humoral response at the end of a 2-dose mRNA vaccine regimen in these populations. The additional third dose allowed increasing this response in non-responders or low responders to two doses; however, the levels were still lower than those observed in the controls.
Author contributions
PL and LW: original draft; LN, BB, JYB, MH, ET, ML BL, JDL, JM, SNQ, JPS, BT, AT, JFV, FV, SC, PV, ET, BP, XL; PL, LW and OL: writing – review and editing, conceptualization; PL, LN, BB, JYB, MH, ET, ML, BL, JDL, JM, SNQ, JPS, BT, AT, JFV, FV, SC, PV, ET, BP, and XL: investigation; LW and MC: methodology; LW and MC: formal analysis; PL, LW, LE, AL, ET, XL and OL: project administration.
Funding
Funding was received from Agence Nationale de Recherche contre le Sida et les Maladies Infectieuses Emergentes (ANRS|MIE) and the French ministries (Ministère des Solidarités et de la Santé and Ministère de l’Enseignement Supérieur, de la Recherche et de l’Innovation).
Transparency declaration
PL has received payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from AstraZeneca, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, Pfizer and Sanofi Pasteur and support for attending meetings and/or travel from Pfizer and Sanofi Pasteur. JM has received payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from AbbVie, Biogen, BMS, Boerhinger Ingelheim, Galapagos, GSK, Fresenius Kabi, Lilly, Mylan, Novartis, Pfizer and Sanofi and grants outside the submitted work from Lilly and Novartis. JPS has received payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from Pfizer and AstraZeneca. OL has received payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from Sanofi Pasteur, Pfizer and Janssen; non-financial support from Sanofi Pasteur, Pfizer, Janssen and GlaxoSmithKline and a grant from GlaxoSmithKline. The other authors declare having no conflict of interest.
Acknowledgements
The authors would like to thank all participants and the dedicated staff of the local centres and their respective biological resource centres, who continuously participated with enthusiasm in the cohort and rendered this research possible. Without their on-going participation, a cohort of this magnitude would not have been possible. We thank the biobank ANRS SC10 US109 (centre méthodologique de gestion des essais cliniques) INSERM for its huge on-going contribution to this project and the COVIREIVAC Network team for its support. We thank ANRS | Maladies infectieuses émergentes and its coronavirus disease 2019 scientific committee and Comité Ad-hoc de Pilotage National des Essais Therapeutiques (CAPNET), the national steering committee for clinical trials and other research on coronavirus disease 2019 for their support for this study. Finally, we also thank the ANRS|MIE and the French ministries (Ministère des Solidarités et de la Santé and Ministère de l’Enseignement Supérieur, de la Recherche et de l’Innovation) for their generous on-going financial support.
Editor: C. Roy
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.10.009.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Galmiche S., Luong Nguyen L.B., Tartour E., de Lamballerie X., Wittkop L., Loubet P., et al. Immunological and clinical efficacy of COVID-19 vaccines in immunocompromised populations: a systematic review. Clin Microbiol Infect. 2021;28:163–177. doi: 10.1016/j.cmi.2021.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee A.R., Wong S.Y., Chai L.Y., Lee S.C., Lee M.X., Muthiah M.D., et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376 doi: 10.1136/bmj-2021-068632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Fusco M., Lin J., Vaghela S., Lingohr-Smith M., Nguyen J.L., Scassellati Sforzolini T., et al. COVID-19 vaccine effectiveness among immunocompromised populations: a targeted literature review of real-world studies. Expert Rev Vaccines. 2022;21:435–451. doi: 10.1080/14760584.2022.2035222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun J., Zheng Q., Madhira V., Olex A.L., Anzalone A.J., Vinson A., et al. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med. 2022;182:153–162. doi: 10.1001/jamainternmed.2021.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Fusco M., Moran M.M., Cane A., Curcio D., Khan F., Malhotra D., et al. Evaluation of COVID-19 vaccine breakthrough infections among immunocompromised patients fully vaccinated with BNT162b2. J Med Econ. 2021;24:1248–1260. doi: 10.1080/13696998.2021.2002063. [DOI] [PubMed] [Google Scholar]
- 6.Embi P.J., Levy M.E., Naleway A.L., Patel P., Gaglani M., Natarajan K., et al. Effectiveness of 2-dose vaccination with mRNA COVID-19 vaccines against COVID-19–Associated hospitalizations among immunocompromised adults—Nine States, January–September 2021. MMWR. 2021;70:1553–1559. doi: 10.15585/mmwr.mm7044e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker E.P., Desai S., Marti M., Nohynek H., Kaslow D.C., Kochhar S., et al. Response to additional COVID-19 vaccine doses in people who are immunocompromised: a rapid review. Lancet Glob Health. 2022;10 doi: 10.1016/S2214-109(21)00593-3. e326–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loubet P., Wittkop L., Tartour E., Parfait B., Barrou B., Blay J.Y., et al. A French cohort for assessing COVID-19 vaccine responses in specific populations. Nat Med. 2021;27:1319–1321. doi: 10.1038/s41591-021-01435-1. [DOI] [PubMed] [Google Scholar]
- 9.Feng S., Phillips D.J., White T., Sayal H., Aley P.K., Bibi S., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamar N., Abravanel F., Marion O., Romieu-Mourez R., Couat C., Del Bello A., et al. Assessment of 4 doses of SARS-CoV-2 messenger RNA-based vaccine in recipients of a solid organ transplant. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caillard S., Thaunat O., Benotmane I., Masset C., Blancho G. Antibody response to a fourth messenger RNA COVID-19 vaccine dose in kidney transplant recipients: a case series. Ann Intern Med. 2022;175:455–456. doi: 10.7326/L21-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brill L., Rechtman A., Zveik O., Haham N., Oiknine-Djian E., Wolf D.G., et al. Humoral and T-cell response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol. 2021;78:1510–1514. doi: 10.1001/jamaneurol.2021.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Disanto G., Sacco R., Bernasconi E., Martinetti G., Keller F., Gobbi C., et al. Association of disease-modifying treatment and anti-CD20 infusion timing with humoral response to 2 SARS-CoV-2 vaccines in patients with multiple sclerosis. JAMA Neurol. 2021;78:1529–1531. doi: 10.1001/jamaneurol.2021.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.König M., Torgauten H.M., Tran T.T., Holmøy T., Vaage J.T., Lund-Johansen F., et al. Immunogenicity and safety of a third SARS-CoV-2 vaccine dose in patients with multiple sclerosis and weak immune response after COVID-19 vaccination. JAMA Neurol. 2022;79:307–309. doi: 10.1001/jamaneurol.2021.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louapre C., Collongues N., Stankoff B., Giannesini C., Papeix C., Bensa C., et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77:1079–1088. doi: 10.1001/jamaneurol.2020.2581. https://doi.or/10.1001/jamaneurol.2020.2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.