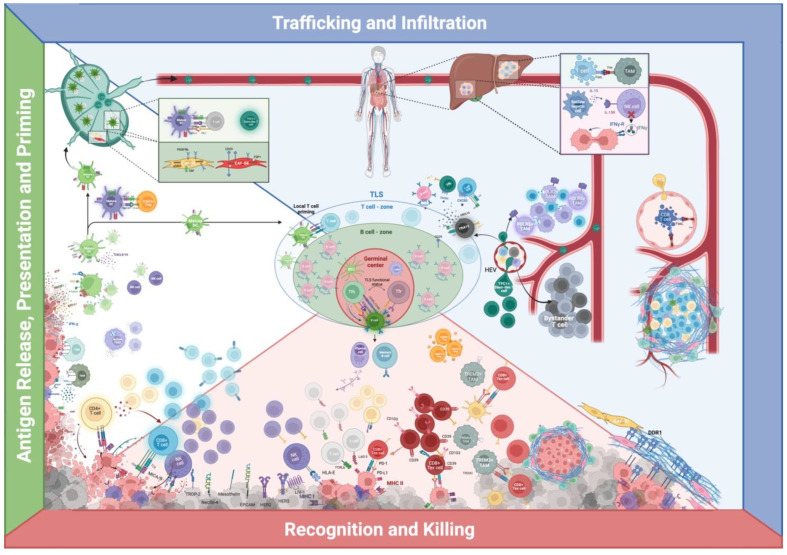
Figure 3.
The cancer-immunity cycle and multiplexed in situ spatial protein profiling of relevant cellular interactions and niches in breast cancer. Green—antigen release, presentation and priming: tumor-associated antigens (TAAs) are captured by innate immunity, whose activation is enhanced by damage-associated molecular patterns (DAMPs) and limited by mechanisms altering TME’s adjuvanticity, as depicted in Figure 2. Activated dendritic cells (DCs) can therefore migrate to tumor-draining lymph nodes (TDLN), where they can prime naïve T cells. Regulatory FOXP3+ T cells (Treg) can target migrating DCs and thus limit T cell priming. NK cells can favor DCs’ recruitment and physically interact with DCs and, in TDLN, DCs’ interaction with T cells can foster a transiently activated T cell phenotype or stimulate a stem-like T cell phenotype. Different subtypes of cancer-associated fibroblasts (CAFs) can populate TDLN and influence the incidence and pattern of metastasis, as CAF-S1 and S4. DCs can prime T cells in tertiary lymphoid structures (TLS), thus bypassing TDLN. Blue—trafficking and infiltration: cellular components of the immune system must access and repopulate the TME to exert cancer-immune control. On the far right: cancer cells can limit CD8+ T cells’ endothelial access by inducing the expression of FASL on tumor-associated endothelial cells (TA-EC), meanwhile positively regulating regulatory FOXP3+ T cell (Treg) passage. Cancer cells can alter T cells intrastromal motility by directly altering the extracellular matrix (ECM)—such as with DDR1 expression—or indirectly modulate ECM-structure by subjugating different CAFs subpopulations. On the right: CD8+ T cells engage in peri-vascular niches with FOLR2+ tumor-associated macrophages (TAMs); TCF+ stem-cell like T cells can access the TME through specialized high endothelial venules (HEVs). Further, in grey, the bystander T cells, whose role in BC is still unclear. In the middle—human figure: TME profiling of metastatic sites. > On the upper portion: Cells can indirectly limit T cell infiltration by systemically depleting T cells through TAMs-mediated siphoning of tumor-specific CD8+ T cells in hepatic metastasis. > On the lower portion: hepatic stellate cells’ interaction with NK cells can limit their cancer control ability. TLS present the unique potential of locally stimulating both T cell priming and activation, B cell maturation, and natural antibody production. CXCL13+ follicular T cell helpers (TfhX13) can coordinate CXCR5 positive immune cells into aggregating in multi-cellular structures, which could be the precursors of TLS. Red—recognition and killing: innate and acquired immune cells can find and destroy cancer cells by leveraging their antigenicity (MHC class I/II expression), which is counterbalanced by many immune-evasive mechanisms described in the antigenicity section in Figure 1. Even when T cells are allowed to reach the tumoral bed, cancer cells can express PD-L1 and directly limit the activity of T cells expressing PD-1 or hide from them in niches constructed by CAFs; furthermore, T cells can be physically surrounded by TREM2+ TAMs and other APCs, which can alter their killing endeavor. Created with BioRender.com.