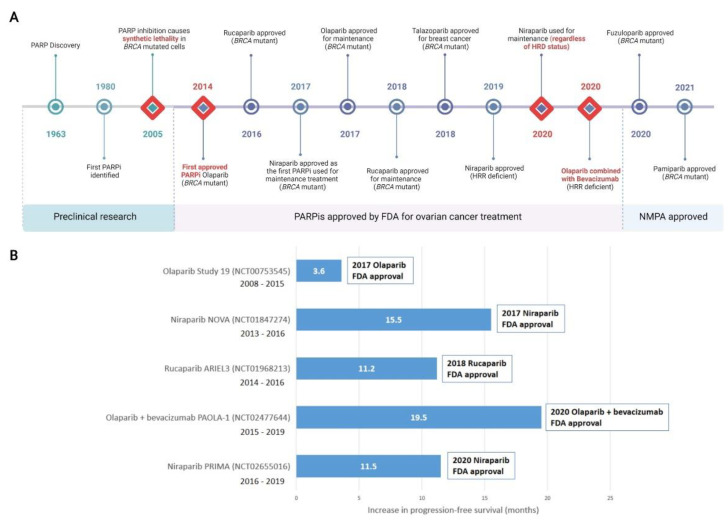
Figure 2.
Discovery of PARP inhibitors and their introduction to the clinic. (A) Milestones in the discovery of PARP and clinical adoption of PARPis are recorded over time. Major milestones are indicated with a red diamond. All milestones are specific to the treatment of ovarian cancer, with the exception of talazoparib which was first approved for metastatic breast cancer and not currently approved for ovarian cancer. (B) Selected landmark clinical trials investigating progression-free survival (PFS) that were instrumental in clinical approval of PARPis, including in combination therapy, are shown. Increased PFS in treatment compared to placebo arms are represented. Additional months of PFS for treatment versus placebo groups are reported for the whole cohort (NCT00753545); BRCA mutant patients (NCT01847274 and NCT01968213); HRD positive patients including BRCA mutant (NCT02477644); HRD positive patients (NCT02655016). Detailed clinical trial information is reported in Supplementary Tables S1 and S2. For trials noted as active but not recruiting, the primary completion date for data collection is noted. FDA, Food and Drug Administration (US); NMPA, National Medical Products Administration (China). HRD, homologous recombination deficiency; HRR, Homologous Recombination Repair; PARPi, PARP inhibitor. Created with Biorender.com.