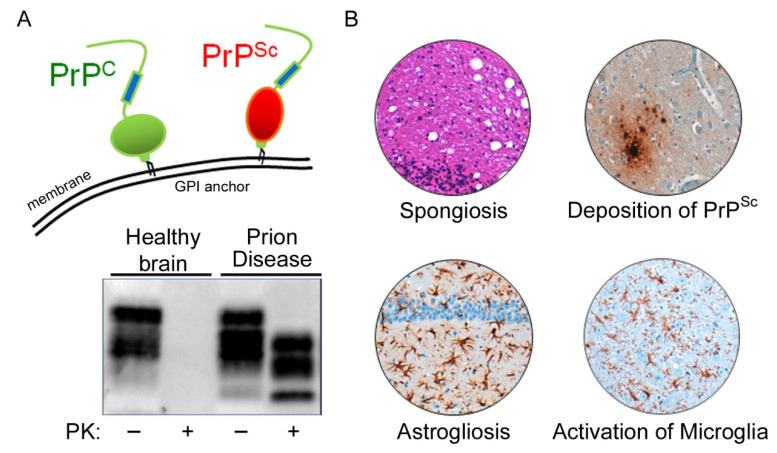
Figure 1.
The cellular prion protein is misfolded in prion diseases. (A) A glycosylphosphatidylinositol (GPI) anchor tethers the cellular prion protein (PrPC; schematic in green) to the outer leaflet of the plasma membrane. In prion disease, PrPC is conformationally converted into its disease-associated isoform PrPSc (schematic in red). Misfolded PrPSc is more stable against proteolytic digestion and can be detected in prion disease but not in healthy brain tissue after proteinase K (PK) digestion by Western blotting. (B) Prion disease histopathological hallmarks include spongiform changes (hematoxylin and eosin (H&E) staining, see vacuolation), deposition of aggregated protein (misfolded PrPSc), astrogliosis (GFAP), and microglial activation (IBA1).