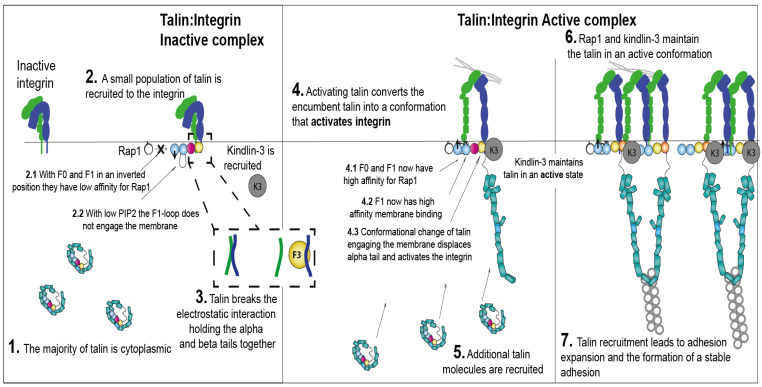
Figure 6.
A model of integrin activation by talin-kindlin-Rap1. (1). The majority of talin is in the cell cytoplasm. Integrin adopts a conformation with bent ectodomain and low affinity for ligands. (2). Integrin activation is initiated by a small population of talin being recruited to the integrin. Talin adopts a conformation with inverted F0-F1, in which F0 has low affinity for the membrane-bound Rap1 and F1-loop has low affinity for PIP2. (3). Talin F3 binding to integrin β leads to breaking of the inner membrane clasp. (4). The F0F1 subdomains are inverted, allowing for F0 and F1 to engage Rap1 and PIP2. Displacing the α tail leads to separation of the integrin α and β transmembrane domains and conversion of integrin into the active conformation (extended high affinity). Kindlin-3 cooperates with talin and maintains talin-integrin in an active state. (5). More PIP2 and active Rap1 are generated at the plasma membrane, which recruits additional talin molecules to form the Rap1-talin-integrin-membrane complex. (6). The active integrin conformation is maintained by a complex containing Rap1, talin-1, kindlin-3 and integrins. This complex is linked to the actin cytoskeleton through talin. Kindlin-3 may also contribute to the clustering of integrins at this stage. (7). Talin recruitment and integrin activation engaging ligands leads to more molecule recruitment, resulting in adhesion expansion, strengthening and stabilization.