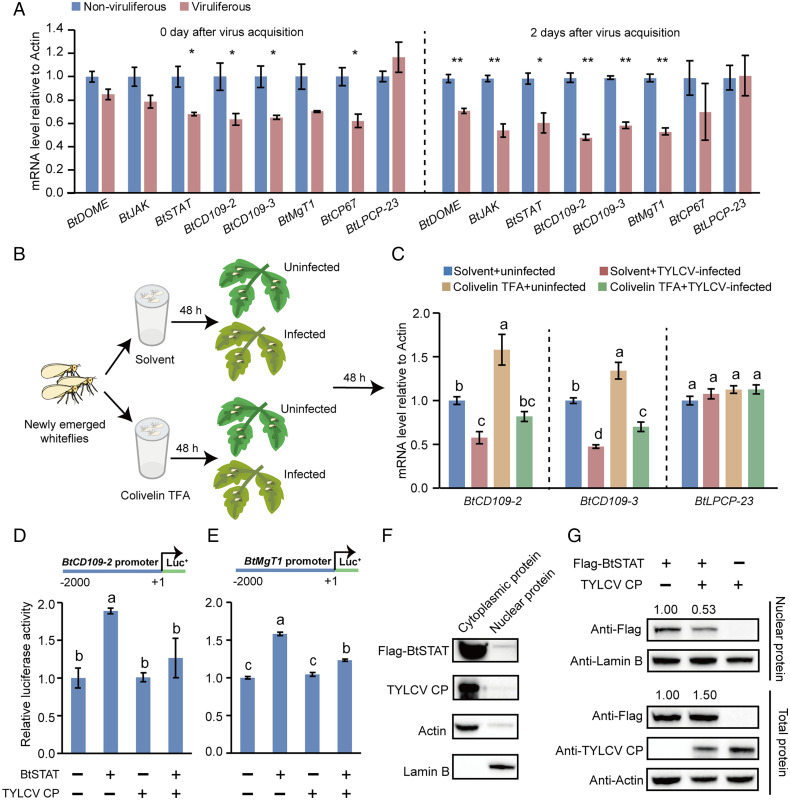
Fig. 4.
TYLCV infection inhibits the JAK/STAT pathway. (A) Effect of TYLCV infection on the expression of BtDOME, BtJAK, BtSTAT, and BtSTAT-regulated genes BtCD109-2, BtCD109-3, BtMgT1, and BtCP67 in whiteflies as detected by qRT-PCR. The mRNA levels of BtLPCP-23, which is not regulated by BtSTAT, were detected in parallel as a control. Whiteflies were sampled at the time points as indicated after transfer onto cotton plants following a 48-h AAP on TYLCV-infected or uninfected tomato plants. Data are represented as mean ± SEM from three independent experiments with 40 whiteflies in each replicate; *P < 0.05 and **P < 0.01 (independent-samples t test). (B and C) Effect of TYLCV infection on the expression of BtSTAT-regulated genes BtCD109-2 and BtCD109-3 in colivelin TFA– or solvent (H2O)-treated whiteflies. The mRNA levels of BtLPCP-23 were detected in parallel as a control. Data are represented as mean ± SEM from three independent experiments with 40 whiteflies in each replicate; P < 0.05 (one-way ANOVA, least significant difference [LSD] test). (D and E) TYLCV CP suppresses BtSTAT-mediated transactivation. HEK293 cells were transfected with the expression vectors for TYLCV CP and/or BtSTAT together with the reporter construct BtCD109-22kb-Luc (D) or BtMgT12kb-Luc (E). Treatments with empty expression vector served as controls. A Renilla luciferase reporter construct was cotransfected in each well as an internal reference. Data represent normalized luciferase activity (firefly/Renilla). Data represent mean ± SEM from three independent experiments; P < 0.05 (one-way ANOVA, LSD test). (F and G) TYLCV CP blocks BtSTAT nuclear translocation. (F) Immunoblotting analysis of Flag-BtSTAT, TYLCV CP, actin, and lamin B in the cytoplasm and nucleus of HEK293 cells coexpressing Flag-BtSTAT and TYLCV CP. (G) HEK293 cells were transfected with the expression vectors for TYLCV CP and/or BtSTAT. Total proteins and nuclear proteins were extracted and analyzed by immunoblotting using Flag, TYLCV CP, actin, and lamin B antibodies. Lamin B served as a loading control for nuclear protein, while actin served as a loading control for total protein.