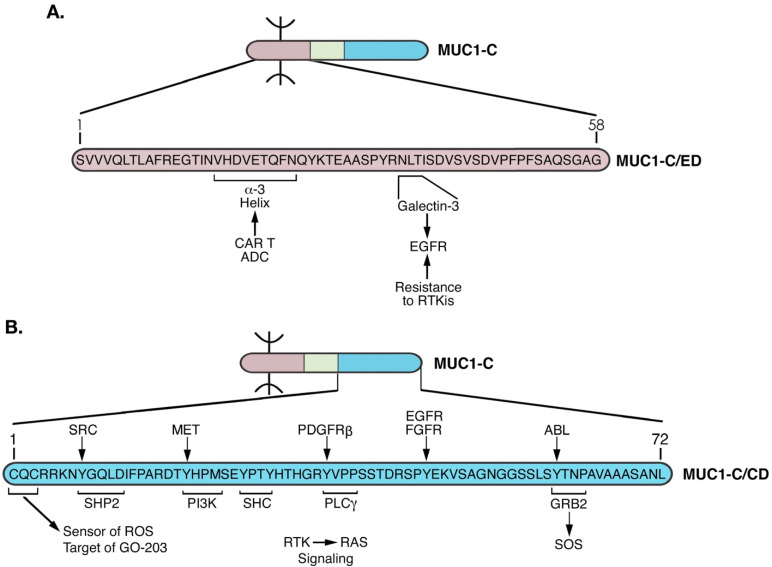
Figure 9.
Targeting the MUC1-C extracellular and cytoplasmic domains for disruption of auto-inductive nodes and elimination of CSCs. (A). Antibody 3D1 generated against the MUC1-C/ED alpha-3 helix has been developed for (i) allogeneic CAR T cells that are under clinical evaluation, and (ii) ADCs that are being advanced with IND-enabling studies by the NCI NExT Program. MUC1-C forms complexes with EGFR at the cell membrane that are mediated by galectin-3 [44]. In this way, MUC1-C contributes to EGFR activation and resistance to EGFR inhibitors [101]. Antibodies generated against the alpha-4 helix are being developed to block the MUC1-C/ED interaction with galectin-3 and thereby inhibit constitutive MUC1-C-driven RTK activation. (B). The MUC1-C/CD CQC motif is necessary for MUC1-C homodimerization and function as an oncoprotein. Targeting the MUC1-C CQCRRKN region with the GO-203 inhibitor blocks interactions with TCF4 [61], TAK1 [69] and JAK1 [82]. GO-203 treatment also inhibits the interactions of MUC1-C with STAT3 [82] and NF-κB [77]. As a result, targeting the MUC1-C CQC motif disrupts auto-induction of MUC1-C NODES 1–3. Ongoing work is addressing another MUC1-C node that may be of importance for RTK→RAS signaling in cancer. In this regard, MUC1-C forms complexes with effectors of the RTK→RAS pathway that include PI3K [102], SHC, PLCγ and GRB2/SOS [103].