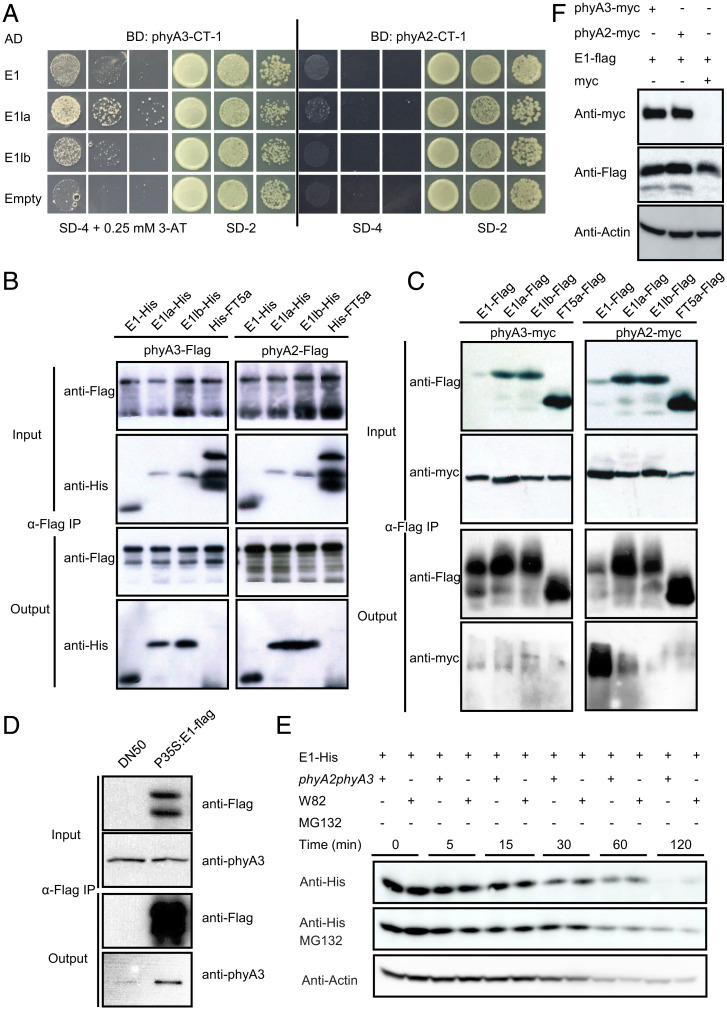
Fig. 4.
Protein interactions of E1 and its homologs with phyA2 and phyA3. (A) E1 and its homologs interact with phyA3-CT-1 (454 aa–1130 aa), but not with phyA2-CT-1 (447 aa–1123 aa) in yeast. Yeast cells transformed with indicated genes were selected on SD-2 (lacking Leu and Trp) and SD-4 (lacking Ade, His, Leu, and Trp) media with indicated 3-AT concentration. (B) phyA2 and phyA3 can pull down E1, E1la, E1lb. E1-His, E1la-His, E1lb-His, and His-FT5a proteins were expressed in E. coli, and phyA2-flag and phyA3-flag protein were expressed using an in vitro translation system. Purified proteins were used for the pull-down assay. phyA2-flag and phyA3-flag were detected with anti-flag antibody, and E1-His, E1la-His, E1lb-His, and His-FT5a protein were detected with anti-His antibody. (C) E1 and its homologs interact with phyA2 and phyA3 in Nicotiana benthamiana leaves in a co-IP assay. phyA2-myc and phyA3-myc were detected with anti-myc antibody, and E1-flag, E1la-flag, E1lb-flag, and FT5a-flag protein were detected with anti-flag antibody. (D) E1 interacts with phyA3 in soybean leaves in a co-IP assay. (E) Cell free in vitro degradation system indicating that E1-His is stabilized in protein extracts from W82 plants. Anti-actin was used as a sample loading control. (F) Transient expression in N. benthamiana leaves demonstrating that phyA2 and phyA3 stabilize E1 in plants. Anti-actin was used as a sample loading control.