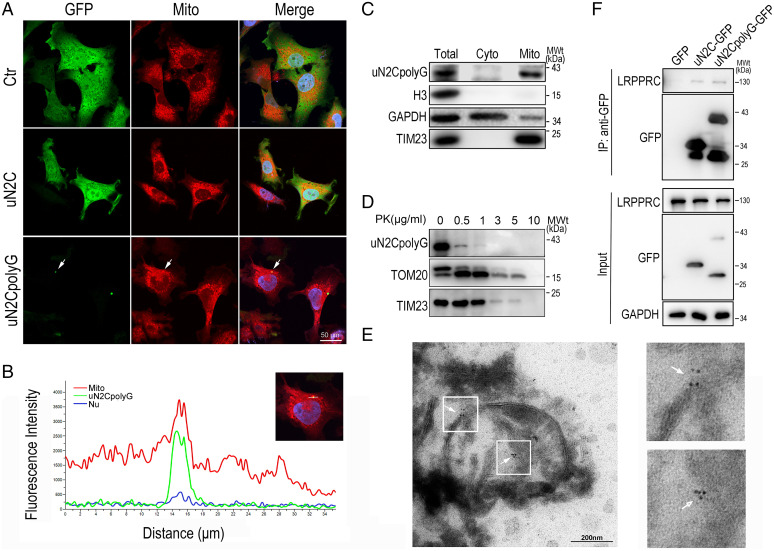
Fig. 5.
Colocalization of uN2CpolyG and mitochondria in a SH-SY5Y cell model and NIID brain tissue. (A) Representative immunofluorescence microscopy images of SH-SY5Y cells expressing GFP, uN2C-GFP, and uN2CpolyG-GFP at 24 h posttransfection. Mitochondria were partially colocalized with uN2CpolyG (indicated by arrows). (B) Line scan analysis of the merged images showed uN2CpolyG was colocalized with mitochondria in SH-SY5Y cells expressing uN2CpolyG-GFP. (C) Isolated mitochondria were prepared from SH-SY5Y cells expressing uN2CpolyG-GFP. The mitochondrial purity was confirmed by the detection of mitochondrial TIM23 and the absence of the cytoplasmic proteins such as GAPDH or nuclear protein Histone H3. The uN2CpolyG protein was localized to mitochondria. (D) Isolated mitochondria from SH-SY5Y cells expressing uN2CpolyG-GFP were subjected to digestion with PK. The uN2CpolyG protein, the OMM protein, TOM20, and the inner mitochondrial membrane (IMM) protein TIM23 were detected. (E) Immuno-EM images of brain tissues from the NIID patient showed uN2CpolyG-immunostaining signals associated with mitochondria labeled with 6-nm immunogold particles (indicated by arrows; uN2CpolyG is labeled by a 4D12-specific antibody). (F) uN2CpolyG-LRPPRC interaction was detected by coIP assay. Western blot was performed using corresponding specific antibodies following immunoprecipitation of cell lysates with anti-GFP. Cyto, cytoplasm; IP, immunoprecipitation.