Figure 2. PNT disrupts proteasomal function and changes MHC I peptidome of tumor cells.
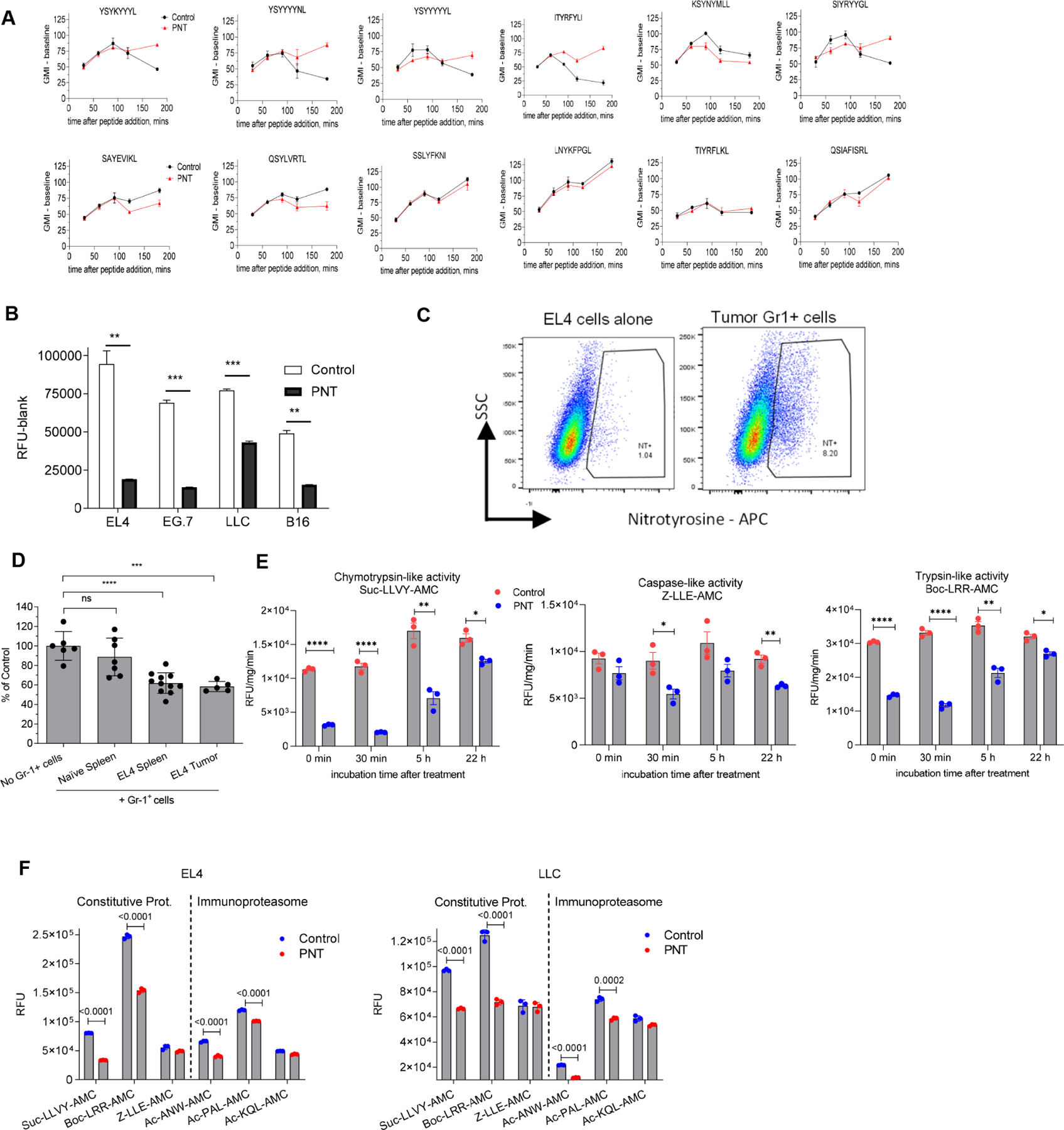
A. The effect of peptides pretreatment with PNT on the MHC I binding ability. 40 μg of specified peptides were either left untreated (Untr) or pretreated with PNT (2.5 mM). Reaction was stopped by diluting PNT to 0.125 mM (PNT). The binding assay was conducted with 10 μg/ml final concentration of peptide. Fluorescence of background (cells without peptides) was subtracted. Mean ± SD are shown. (n=2 for control and n=3 for PNT groups). B. Indicated tumor cells were treated with 2.5 mM PNT for 5 min and proteasomal activity was measured in the lysates (n=3). Mean ± SD are shown. ** - p<0.01; *** - p<0.001 in two-sided Student’s t-test. C. EL4 cells were co-cultured with Gr-1+ splenic cells from tumor-bearing mice for 18 h and analyzed for NT presence on cell membrane. D. Gr-1+ cells isolated from spleens and tumors of TB or control mice were cultured with EL4 tumor cells for 18 h. Gr-1+ cells were depleted by magnetic beads and proteasomal activity was measured in the tumor cells. Mean ± SD are shown. n=5, *** - p<0.001, **** - p<0.0001 in one-way ANOVA test with correction for multiple comparisons. E. EG.7 cells were treated at indicated time with vehicle (control) or 2.5 mM PNT. Chymotrypsin, trypsin and caspase-like activities were measured using the corresponding fluorogenic substrate. Mean ± SD are shown. N=3. P values were calculated in unpaired Student’s t-test. **-p<0.01; ***-p<0.001; **** - p<0.0001. F. EL4 and LLC tumor cells were treated with 2.5 mM PNT for 5 min on ice. Proteasomal activity was measured with the use of indicated fluorogenic substrates. n= 3. P values were calculated in two-sided Student’s t-test. Mean and SD are shown. See also Figure S4.
