Figure S5.
Testing intrinsic promoter activities, bisulfite conversion cloning and generation and analysis of DNMT3A/B knockouts, related to Figures 4 and 5
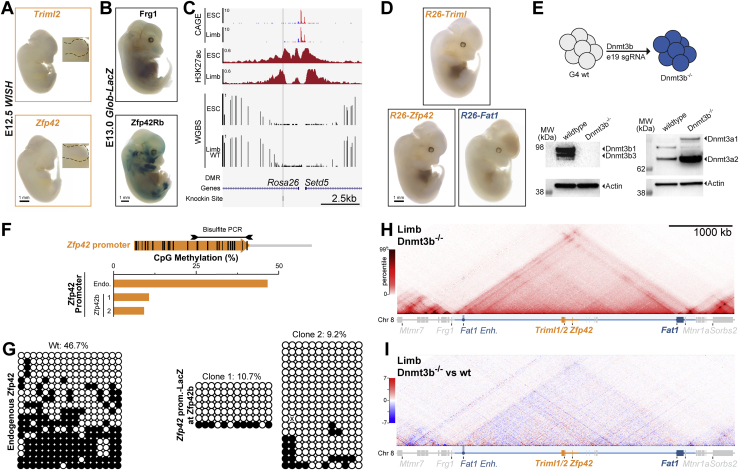
(A) Triml2 and Zfp42 WISH stainings in E12.5 embryos. Scale bar is 1 mm.
(B) Staining from β-globin LacZ sensors integrated at Frg1 and Zfp42Rb in E13.0 embryos. n = 4–10 stained embryos per position.
(C) Zoom of Rosa26 safe harbor locus with CAGE, H3K27ac ChIP-seq and WGBS shown. Sensor integration site is indicated by the gray bar with insert transcription orientation matching Rosa26.
(D) LacZ stainings from E12.5 embryos with sensors driven by indicated promoters integrated at the Rosa26 locus.
(E) Strategy for Dnmt3b knockout in ESC clones with western blot confirmation shown below. DNMT3A increases following loss of DNMT3B.
(F) Schematic of bisulfite conversion cloning strategy with quantification of methylated CpGs at the endogenous or transplanted Zfp42 promoter in E11.5 limbs.
(G) Corresponding lollipop diagrams of Zfp42 promoter methylation (black methylated and white unmethylated CpGs).
(H and I) cHi-C from E11.5 DNMT3B−/− limb buds (H) with subtraction to wild type shown below (I).