Abstract
Background
The coronavirus disease 2019 (COVID-19) has outbroken into a global pandemic. The death rate for hospital patients varied between 11% and 15%. Although COVID-19 is extremely contagious and has a high fatality rate, the amount of knowledge available in the published literature and public sources is rapidly growing. The efficacy of convalescent plasma (CP) therapy for COVID-19 is controversial.
Objective
This meta-analysis was designed to assess the efficacy of CP therapy for COVID-19 through a literature survey.
Methods
Until August 30, 2021, a literature search was undertaken in Pubmed, Embase, Web of Science, Cochrane Central Register of Controlling Trials (Central), and China National Knowledge Infrastructure databases. The Risk Ratio (RR) and 95% confidence intervals (CIs) were pooled using a fixed or random effect model in dichotomous data. Mean difference (MD) and 95% confidence intervals (CIs) were pooled using a fixed or random effect model in continuous data. Studies with missing or unsuitable data were presented descriptively in the outcomes.
Results
In total, thirteen randomized controlled trials (RCTs) were selected for the present meta-analysis, which included a total of 13232 participants. Our results revealed that the CP group has lower mortality compared to the control group, and there was a statistically significant difference (RR: 0.70, 95% CI: 0.55, 0.89, Z = 2.92, P = 0.004 < 0.01); other secondary outcomes such as the shortness of breath symptom improved significantly in CP group (RR:1.48, 95% CI: 1.13, 1.93, Z = 2.85, P = 0.004 < 0.01), as well as Interleukin-6 (IL-6) (MD: −4.46, 95% CI: −8.28, −0.63, Z = 2.28, P = 0.02 < 0.05) and Ferritin (MD: −447.68, 95% CI: −501.75, −393.6, Z = 16.23, P < 0.00001) are reduced significantly in CP group. However, there was no statistically significant change in the ventilator withdrawal rate, imaging results improvement, or days to hospital discharge. There was also no substantial difference in viral nucleic acid negative conversion rate and neutralizing antibody-positive conversion rate, as well as the incidence of adverse reactions.
Conclusions
The safety and potential efficacy of convalescent plasma therapy offer a promising treatment strategy for COVID-19. CP therapy can reduce mortality and improve breath and inflammatory cytokines IL-6 and Ferritin in COVID-19 with no significant increase in adverse reactions. However, it does not affect improving virology indicators. In summary, more high-quality clinical trials are needed to verify the conclusion of the present study.
1. Introduction
In December 2019, several patients in Wuhan, Hubei Province of China, were diagnosed with pneumonia of unknown etiology [1]. In January 2020, the Chinese Center for Disease Control and Prevention (CDC) confirmed a patient's throat swab sample as the source of Novel Coronavirus, which the World Health Organization (WHO) subsequently designated as coronavirus disease 2019 (COVID-19). Novel coronavirus pneumonia, also known as COVID-19, is a viral respiratory syndrome caused by SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus Type 2) [2]. On March 11, 2020, the WHO announced that the spread of coronavirus disease in 2019 will pose persistent and significant difficulties to the global community.
SARS-CoV-2 infection not only influenced human beings' daily activities [3] but also damaged organs. Evidence showed that SARS-CoV-2 directly or indirectly activates inflammasomes. The inflammatory cytokines lead to pyroptosis, an inflammatory form of cell death, amplify the destructive tissue damage via endothelial dysfunction and vasodilation, promoting endothelins and then resulting in tissue damage and developing into a variety of clinical symptoms [4]. In extreme circumstances, this inflammatory response can cause numerous organ failures [5]. When faced with this scenario, we must act swiftly to present therapeutic choices. Despite advances in vaccination and drug research, including nanotechnology-based drug [6]and natural bioactive molecules [7, 8], unfortunately, due to the genetic diversity and rapid evolution of this novel coronavirus, there is still no special effective treatment has been found to contain the disease [9–11]. General supportive care for novel coronavirus pneumonia is currently the only choice [12]. As is known to all, passive immunity has played an important role in treating infectious diseases [13]. The most recent anti-SARS-CoV-2 treatment, AZD7442, was developed using antibodies isolated from B cells collected from SARS-CoV-2 infected patients and is effective. Still, it is so expensive that is difficult to become widely used [14].
Convalescent plasma (CP) therapy is a form of passive immunity in which antibody-rich blood is collected from recovered patients and infused into other patients after treatment. CP therapy was evaluated in treating Severe Acute Respiratory Syndrome (SARS) in 2003 [15], followed by the Middle East respiratory syndrome (MERS) epidemic [16] and the Ebola epidemic [17]. There is evidence that receptor binding domain-specific antibodies with strong anti-viral activity have been found in the convalescent plasma survivors of novel coronavirus pneumonia [18]. In the first few days of the novel coronavirus pneumonia pandemic, due to a seemingly reasonable mechanism of action, CP generated great enthusiasm [19].
Recently, reports of improvement results of novel coronavirus pneumonia after CP transfusion have been recorded in randomized clinical trials [20, 21]. However, there is still disagreement over the effectiveness of convalescent plasma therapy for COVID-19. Data on the performance of CP in the treatment of novel coronavirus pneumonia that has been reported will be summarized through this systematic review and meta-analysis.
2. Methods
2.1. Inclusion and Exclusion Criteria
Studies eligible for inclusion must meet the following criteria [22]: (1) include patients diagnosed with COVID-19; (2) only select the published type of RCT; (3) choose CP therapy for intervention; (4) compare the CP with the standard of care (intervention arm) with a standard of care alone (control arm); (5) complete data on intervention group and control group. We excluded review articles, case reports, and case series.
2.2. Search Strategy and Study Selection
The reporting of our systematic review and meta-analysis follows the criteria for recommended reporting items for systematic reviews and meta-analyses (PRISMA) [23]. We searched for COVID-19 and COVID-19 serotherapy in Pubmed, Embase, Web of Science, Cochrane Central Register of Controlling Trials (Central), and China National Knowledge Infrastructure through August 30, 2021. Studies that are already included can be used in various languages. The two authors independently reviewed the abstracts of all publications to determine their eligibility.
2.3. Study Outcome Measures
The primary outcome is mortality, which refers to all causes of mortality from the time of randomization to the clinical observation endpoint. Secondary outcomes are: (1) clinical improvement rate includes the rate of improvement of shortness of breath, the rate of taking off the ventilator, the improvement of imaging results, the degree of improvement in inflammatory indicators, the degree of improvement of other indicators; (2) incidence of adverse reactions were defined as causes discomfort or pain after treatment that is incompatible with the purpose of treatment; (3) days to hospital discharge that was defined as the number of days from admission to discharge; (4) The improvement rate of virology indicators was analyzed from viral nucleic acid negative conversion rate and neutralizing antibody-positive conversion rate. The viral nucleic acid negative conversion rate was defined as PCR results of COVID-19 virus nucleic acid turned negative. The neutralizing antibody-positive conversion rate was defined as the patient's neutralizing antibodies to COVID-19 changed from negative to positive.
2.4. Literature Screening and Data Extraction
Endnote X9 software was used to manage the articles we obtained. Two independent investigators selected titles and abstracts that were no longer supported by literature searches for inclusion in the study. Further analysis of the full text was carried out after reading the abstract and finding that it does not explicitly meet the inclusion criteria. A third investigator was responsible for discussing and resolving any disagreement regarding a study selection. If there was a disagreement in the data extraction, which was done independently by the two reviewers using a standard data extraction form, the arbitrator reviewed and evaluated it [22]. First author, country, year of publication, design, sample size, treatment of patients in the group (CP group and control group), CP dose, outcomes. The information mentioned above belongs to the main components of the extracted data.
2.5. Risk of Bias Assessment
According to the Cochrane Handbook for Systematic Reviews of Interventions, the risk of bias assessment was carried out [22]. The methodological quality of eligible RCTs was evaluated independently through the Cochrane Handbook for Systematic Reviews of Interventions. Specifically, it includes (1) Selection bias: which describes in detail the method for generating randomly assigned sequences; (2) The implementation of bias: concealment of allocation; (3) Measurement bias: blinding of participants, personnel, and outcome assessors; (4) Follow-up of bias: incomplete data on outcome; (5) Reporting bias: selectively reporting favorable results and hiding unfavorable ones; (6) Other biases: conflict of interest, insufficient sample size, unbalanced baseline. Each entry was assessed as “low risk,” “unclear risk,” or “high risk” regarding this statement.
2.6. Statistical Analysis
The statistical evaluation was carried out using Stata 16 and RevMan 5.4. If there were two or more homogenous studies available, we used aggregated data [22]. We calculated the risk ratio (RR), 95% confidence intervals (CI), and P values for dichotomous outcomes. The mean difference (MD), 95% CI, and P values were applied for continuous variables. The I2 statistics were used to evaluate the study heterogeneity. When P ≥ 0.1 and I2 < 50% indicates no heterogeneity present, P < 0.1 or I2 ≥ 50% indicates heterogeneity [22]. If heterogeneity exists, sensitivity analysis was used to find the source of heterogeneity. Finally, Funnel plots were used for assessing publication bias, with P < 0.05 considered significant for publication bias assessment [22].
3. Results
3.1. Literature Selection
Forty-six articles were retrieved and examined after the abstracts and titles of 760 records obtained by the search method were scrutinized. 13 RCTs satisfied the requirements for inclusion. At the same time, the remaining publications were disqualified for lacking control groups, being nonrandomized controlled trials, or being retrospective research. The process of screening the articles is depicted in Figure 1 flowchart.
Figure 1.

Flow diagram of database searches and article selection.
3.2. Characteristics of the Included Studies
Table 1 demonstrates the characteristics of the 13 randomized clinical trials that were considered [24–36]. There were 254 study centers and 13232 individuals, and all had confirmed COVID-19 diagnoses. The language of all papers was English and from June 2020 to August 2021 were the publication years.
Table 1.
Characteristics of the included randomized controlled trials.
| No. | Author | Country | The publishing year | Design | Sample size (T/C) | Patients enrolled condition | Treatment group | Control group | CP dose | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Salman and Mohamed | Egypt | 2020 | Randomized controlled trial, double-blinded | 30 (15/15) | Severe | CP + control group | Supplemental oxygen, noninvasive and invasive ventilation, antibiotic medication, inotrope drugs, renal-replacement therapy, anticoagulants, glucocorticoids, intravenous fluids, interferon, and extracorporeal membrane oxygenation (ECMO) | 250 ml/once, one dose | 2, 3, 5 |
|
| ||||||||||
| 2 | Ali et al. | Pakistan | 2021 | Randomized controlled trial, single-blinded | 50 (40/10) | Severe and critical | C-IVIG + control group | Airway support, anti-viral medication, antibiotics, fluid resuscitation, hemodynamic support, steroids, painkillers, and antipyretics | 0.15, 0.20, 0.25, 0.30 g/kg | 1, 2, 3, 4 |
|
| ||||||||||
| 3 | AlQahtani et al. | Bahrain | 2021 | Randomized controlled trial, open-label | 40 (20/20) | Severe and life-threatening | CP + control group | Control of fever (paracetamol) and possible therapy, including anti-viral medications, tocilizumab, and antibacterial medication | 200 ml/once, two dose | 1, 2, 3, 4 |
|
| ||||||||||
| 4 | Bennett-Guerrero et al. | USA | 2021 | Randomized controlled trial, double-blind | 74 (59/15) | Severe | CP | Standard plasma | 240 ml/once, two dose | 1, 2, 3 |
|
| ||||||||||
| 5 | Gharbharan et al. | The Netherlands | 2021 | Randomized controlled trial, open-label | 86 (43/43) | Moderate, severe, or life-threatening | CP | Standard of care (not specifically described) | 300 ml/once, two dose | 1, 2, 3, 5 |
|
| ||||||||||
| 6 | Peter et al. | UK | 2021 | Randomized controlled trial, open-label | 11558 (5795/5763 | No data reported | CP + control group | Usual care (not specifically described) | Two units (275 mls ± 155 + 75 mls), two-dose | 1, 2, 3 |
|
| ||||||||||
| 7 | Li et al. | China | 2020 | Randomized controlled trial, open-label | 103 (52/51) | Severe and life-threatening | CP + control group | Anti-viral medications, antibacterial medications, steroids, human immunoglobulin, Chinese herbal medicines, and other medications | 4–13 ml/kg | 1, 2, 3, 5 |
|
| ||||||||||
| 8 | Libster et al. | Argentina | 2021 | Randomized controlled trial, double-blind | 160 (80/80) | Mild | CP | Placebo (0.9% normal saline) | 250 ml/once, one dose | 1, 3 |
|
| ||||||||||
| 9 | Pouladzadeh et al. | Iran | 2021 | Randomized controlled trial, single-blind | 62 (31/31) | Severe | CP + control group | Chloroquine phosphate, lopinavir/ritonavir, etc | 500 ml/once, the second the unit was prescribed if no improvement was observed after 24 h | 1, 2, 3, 4 |
|
| ||||||||||
| 10 | O'Donnell et al. | USA, Brazil | 2021 | Randomized controlled trial, double-blind | 223 (150/73) | Severe and critical | CP | Normal control plasma | 200–250 ml/once, one dose | 1, 2, 3, 4 |
|
| ||||||||||
| 11 | Simonovich et al. | Argentina | 2020 | Randomized controlled trial, double-blind | 333 (228/105) | Severe | CP | Placebo (normal saline solution) | 500 ml/once, one dose | 1, 2, 3 |
|
| ||||||||||
| 12 | Agarwal et al. | India | 2020 | Randomized controlled trial, open-label | 464 (235/229) | Moderate | CP + control group | Anti-virals (hydroxychloroquine, remdesivir, lopinavir/ritonavir, oseltamivir), broad-spectrum antibiotics, immunomodulators (steroids, tocilizumab), and supportive management | 200 ml/once, two-dose | 1, 2, 3, 4, 5 |
|
| ||||||||||
| 13 | Rasheed et al. | Iraq | 2020 | Randomized controlled trial, open-label | 49 (21/28) | Critical | CP + control group | Hydroxychloroquine 200 mg twice per day for at least 10 days + azithromycin once 500 mg/day loading dose, followed by 250 mg once per day for 5 days + oxygen therapy + methylprednisolone 40 mg per day | 400 ml/once, one dose | 1, 2, 3, 5 |
T: treatment group. C: control group. CP: convalescence plasma. C-IVIG: hyperimmune anti-COVID-19 intravenous immunoglobulin. Outcome: (1) mortality rate; (2) clinical improvement rate; (3) the incidence of adverse reactions; (4) days to hospital discharge; (5) the improvement rate of virology indicators.
3.3. Risk of Bias within Studies
The bias assessment and summary risk are shown in Figures 2 and 3, respectively. The 13 randomized controlled trials included in this study all adopted appropriate methods for randomization, among which 11 used computer-generated randomization [24, 26–35] and 1 randomized controlled trial selected the same paper cards and numbered them sequentially [36]. The last randomized controlled trial was simple randomization of sequentially numbered opaque sealed envelopes [25]. In terms of ensuring the concealment of the distribution scheme, a total of 10 studies described how to ensure the concealment of the distribution scheme, which was judged to be low risk [24, 25, 27, 29–35]. The remaining 3 studies lacked any description of the allocation hiding method, which was considered to have an unclear bias risk [26, 28, 36]. The following methods respectively concealed the 10 studies: (1) Randomized block design treatment assignment [24]; (2) the study personnel received a sealed opaque envelope with an assignment to intervention or control group [25]; (3) Use SAS software and interactive randomization tools in REDCap (Research Electronic Data Capture) [27, 34]; (4) Random assignment was unstratified and done by local clinical or research staff using a web-based interface with allocation concealment [29]; (5) Treatment assignments were generated using randomly permuted blocks [30, 31, 33]; (6) The individual recruiting the patient (senior physician responsible for therapeutic intervention) contacted the center by phone after the patient is enrolled. The respondent in the center was the second researcher, who had designed a table of the 6-item randomized block by computer and added concealment codes without knowing the patient's medical conditions [32]; (7) A central trail coordination team member provided one RCT with random sequences. The nine RCTs were judged to have high risks of performance deviation and detection deviation [25, 26, 28–30, 32, 33, 35, 36]. All 13 RCTs were judged to be low risk in terms of data integrity and selective reporting of results. None of the 13 randomized controlled trials involved conflicts of interest, small sample sizes, or unbalanced baselines.
Figure 2.

Risk of bias graph.
Figure 3.

Risk of bias summary.
3.4. Analysis of Results
3.4.1. Mortality
At the time of meta-analysis, twelve studies [25–36] compared mortality changes of randomized controlled participants. Those studies in the cluster were tested for heterogeneity (when I2 = 41.5% < 50%, Q test P=0.065 < 0.1), indicating that the heterogeneity among the studies is statistically significant. Therefore, heterogeneity needs to be searched. The sensitivity analysis of the 12 studies found that Peter et al. [29] greatly influenced heterogeneity. After removing this study, the effect variables combined in the meta-analysis were large, as shown in Figure 4. Therefore, after removing the study, the results of heterogeneity again showed that there was no heterogeneity in the remaining 11 studies (when I2 = 11% < 50%, Q test P=0.34 > 0.1, Figure 5), and this meta-analysis was performed using a fixed effect model after exclusion. There was a significant statistical difference (RR: 0.70, 95% CI: 0.55, 0.89, Z = 2.92, P=0.004 < 0.01, Figure 5). According to this, patients who underwent convalescent plasma therapy had a 0.70-times higher mortality risk than those who received standard care or a placebo. This shows that patients with COVID-19 may live longer while receiving convalescent plasma treatment.
Figure 4.
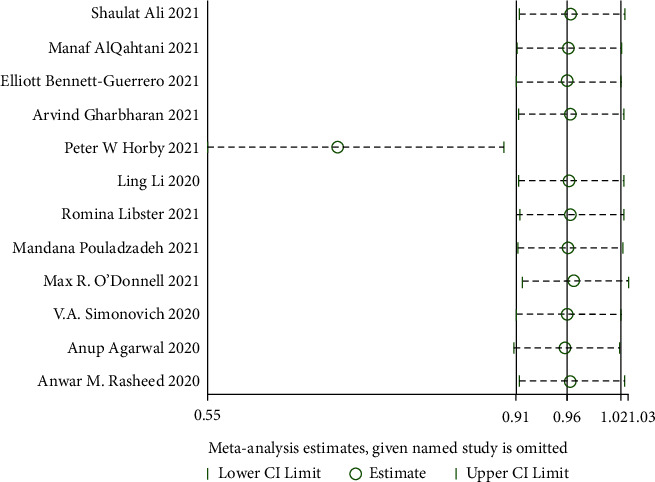
Sensitivity analysis-mortality.
Figure 5.

Forest plot-mortality.
3.4.2. Clinical Improvement Rate
(1) The Rate of Improvement of Shortness of Breath. Three RCTs reported the rate of improvement of shortness of breath [24, 35, 36], and there was substantial statistical heterogeneity among these studies (when I2 = 84.9% > 50%, Q test P=0.001 < 0.1). The sensitivity analysis of the three studies found that the study of Agarwal et al. [35] had a great influence on heterogeneity, as shown in Figure 6. Therefore, after removing this study, the results of heterogeneity again showed that there was no heterogeneity in the remaining two studies (when I2 = 0% < 50%, Q test P=0.45 > 0.1, Figure 7). There was a statistically significant difference between studies (RR:1.48, 95% CI: 1.13, 1.93, Z = 2.85, P=0.004 < 0.01, Figure 7). This demonstrated that patients undergoing convalescent plasma therapy had a breath rate improvement rate that was 1.48 times higher than patients receiving standard care or a placebo. It has been suggested that convalescent plasma therapy can help COVID-19 patients with breathlessness.
Figure 6.

Sensitivity analysis of the rate of improvement of shortness of breath.
Figure 7.

Forest plot for the rate of improvement of shortness of breath.
(2) The Rate of Taking Off the Ventilator. The rate of taking off the ventilator was evaluated in six RCTs [25, 28, 29, 32, 33, 35]. No heterogeneity was observed among these studies (when I2 = 46% < 50%, Q test P=0.1, Figure 8). The fixed effects model was performed directly, revealing no significant difference (RR: 0.99, 95%CI: 0.90, 1.08, Z = 0.28, P=0.78 > 0.05, Figure 8). Therefore, it can be concluded that convalescent plasma therapy is not effective in improving the rate of taking off the ventilator.
Figure 8.

Forest plot of the rate of taking off the ventilator.
(3) The Improvement of Imaging Results. The imaging results were improved in two RCTs [24, 25], and there was a large statistical heterogeneity among the studies (when I2 = 78% > 50%, Q test P=0.03 < 0.1, Figure 9). The random model was used for these analyses. The results showed that there was no significant difference. It is suggested that convalescent plasma therapy is ineffective in improving imaging results (RR:1.77, 95% CI: 0.21, 14.73, Z = 0.53, P=0.60 > 0.05, Figure 9).
Figure 9.

Forest plot demonstrating the improvement of imaging results.
(4) The Degree of Improvement in Inflammatory Indicators.
CRP: The improvement in inflammatory indicators was examined in four RCTs [24, 25, 28, 32]. A strong heterogeneity among studies (when I2 = 98% > 50%, Q test P < 0.1) was observed. As shown in Figure 10, sensitivity analysis cannot identify the study that significantly impacted heterogeneity. Employing the random effect model as a result. The CRP did not differ significantly. According to certain reports, convalescent plasma treatment cannot lower CRP (MD: −40.16, 95% CI: −92.20, 11.89, Z = 1.51, P=0.13 > 0.05, Figure 11).
Ferritin: We analyzed Ferritin in four RCTs [24, 25, 28, 34]. There is strong heterogeneity among studies (when I2 = 96% > 50%, Q test P < 0.1). The sensitivity analysis of the four studies revealed that the study of Simonovich et al. [34] had reported a great influence on heterogeneity, as shown in Figure 12. After removing the study, the results of heterogeneity again showed that there was no heterogeneity in the remaining 3 studies (when I2 = 47% < 50%, Q test P=0.15 > 0.1, Figure 13), using a fixed effect model after exclusion. With a difference of 447.68 between the two groups, there was a significant difference, revealing that patients who received convalescent plasma therapy had lower Ferritin than those who received standard of care or a placebo, implying that convalescent plasma therapy is effective in lowering Ferritin in COVID-19 patients (MD: −447.68, 95% CI: −501.75, −393.6, Z = 16.23, P < 0.00001, Figure 13).
IL-6: IL-6 levels were examined in two RCTs [28, 32], with no heterogeneity among these studies (when I2 = 22% < 50%, Q test P=0.26 > 0.1, Figure 14). There was a significant difference in IL-6 between the CP group and the control group after the fixed model was used. This suggested that participants undergoing convalescent plasma therapy had lower IL-6 levels than those receiving conventional care or placebo, with a 4.46-point difference between the two groups. Convalescent plasma treatment may be useful in lowering IL-6 levels in COVID-19 patients (MD: −4.46, 95% CI: −8.28, −0.63, Z = 2.28, P=0.02 < 0.05, Figure 14)
Figure 10.

Sensitivity analysis for CRP.
Figure 11.

Forest plot for CRP.
Figure 12.
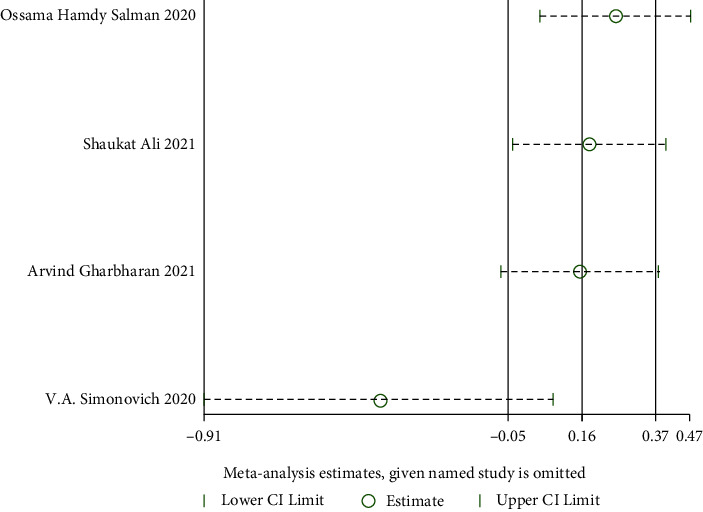
Sensitivity analysis of ferritin.
Figure 13.
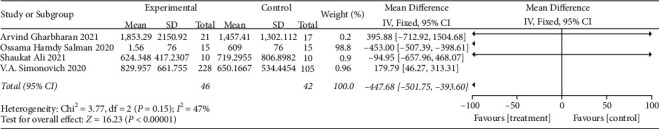
Forest plot for ferritin.
Figure 14.

Forest plot for levels of IL-6.
(5) The Degree of Improvement of Other Indicators
Lymphocyte: The level of lymphocytes was assessed in four RCTs [24, 25, 28, 32], and no significant heterogeneity among these studies was found (when I2 = 22% < 50%, Q test P=0.28 > 0.1, Figure 15). There was no significant difference in lymphocytes. It is suggested that convalescent plasma therapy is not effective in lymphocyte (MD: 0, 95% CI: −0.03, 0.03, Z = 0.14, P=0.89 > 0.05, Figure 15).
D-Dimer: The D-Dimer was evaluated in two RCTs [24, 34], and there was a large statistical heterogeneity among these studies (when I2 = 98% > 50%, Q test P < 0.1, Figure 16), hence the random effects model was used. The findings revealed no significant difference between the CP and control groups. It has been hypothesized that convalescent plasma treatment is ineffective in D-Dimer patients (MD: −518.45, 95% CI: −1754.24, 717.34, Z = 0.82, P=0.41 > 0.05, Figure 16).
Figure 15.

Forest plot for lymphocyte concentration levels.
Figure 16.

Forest plot for D-dimer.
3.4.3. Incidence of Adverse Reactions
Incidence of adverse reactions was tested in thirteen RCTs [24–36], revealing a significant statistical heterogeneity among studies (when I2 = 57% > 50%, Q test P=0.02 < 0.1). The sensitivity analysis of the 13 articles revealed that the studies of Peter et al. [29] and O'Donnell et al. [33] had reported a great influence on heterogeneity, as shown in Figure 17. Therefore, after removing the two studies, there was no heterogeneity in the remaining 11 studies (when I2 = 19% < 50%, Q test P=0.28 > 0.1, Figure 18). The results revealed that there was no significant difference between the CP and control groups. This indicates that convalescent plasma therapy does not affect the occurrence of adverse events in COVID-19 patients (RR:1.55, 95% CI: 1.00, 2.39, Z = 1.95, P=0.051 > 0.05, Figure 18).
Figure 17.
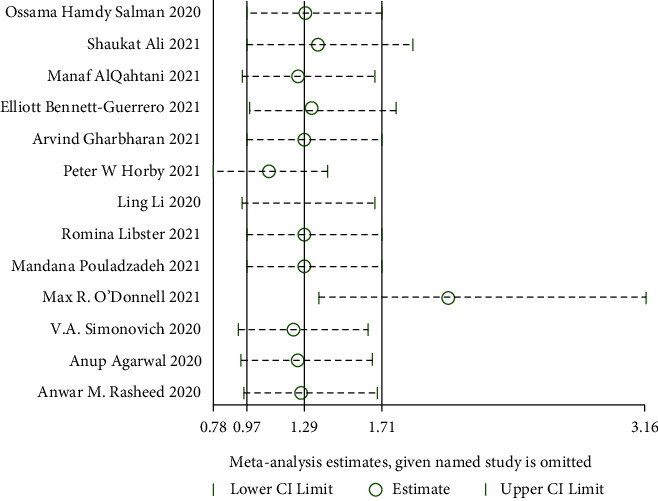
Sensitivity analysis for the incidence of adverse reactions.
Figure 18.

Forest plot for the incidence of adverse reactions.
3.4.4. Days to Hospital Discharge
Six RCTs [25, 26, 28, 32, 33, 35] reported the days to hospital discharge. Heterogeneity test results indicate heterogeneity among these studies (when I2 = 89% > 50%, Q test P < 0.01). Sensitivity analysis was performed on these 6 studies to find heterogeneous causes, and we cannot find the study that greatly influenced heterogeneity, as shown in Figure 19. A random effect model was used for the meta-analysis, and there was no discernible difference. It is suggested that convalescent plasma therapy is ineffective in shortening the days to hospital discharge (MD: −0.06, 95% CI: −2.78, 2.65, Z = 0.04, P=0.96 > 0.05, Figure 20).
Figure 19.

Sensitivity analysis for days to hospital discharge.
Figure 20.

Forest plot for days to hospital discharge.
3.4.5. The Improvement Rate of Virology Indicators
(1) Viral Nucleic Acid Negative Conversion Rate. Viral nucleic acid negative conversion rate analysis was reported in four RCTs [24, 28, 30, 35] with significant heterogeneity (when I2 = 54% > 50%, Q test P=0.114 > 0.1). The sensitivity analysis of the four studies found that one study [35] had reported a significant influence on heterogeneity, as shown in Figure 21. Therefore, after removing the study, the results of heterogeneity again showed that there was no heterogeneity in the remaining three studies (when I2 = 0% < 50%, Q test P=0.05 > 0.1, Figure 22). There was no significant difference in viral nucleic acid negative conversion rate. It is suggested that convalescent plasma therapy is ineffective in viral nucleic acid negative conversion rate (RR: 0.59, 95% CI: 0.32, 1.11, Z = 1.63, P=0.1 > 0.05, Figure 22).
Figure 21.

Sensitivity analysis for the viral nucleic acid negative conversion rate.
Figure 22.

Forest plot of viral nucleic acid negative conversion rate and neutralizing antibody-positive conversion rate.
(2) Neutralizing Antibody-Positive Conversion Rate. Neutralizing antibody-positive conversion rate was tested in two RCTs [24, 36], and there was a statistical heterogeneity among the studies (when I2 = 53% > 50%, Q test P=0.15 > 0.1, Figure 22), so the random effects model was used. The findings revealed no discernible difference between the control group and the patients getting CP therapy. It is hypothesized that convalescent plasma therapy is ineffective in reducing positive antibody conversion rate (RR:6.33, 95% CI: 1.00, 40.1, Z = 1.96, P=0.05, Figure 22).
3.4.6. Publication Bias Detection
Then, we identify publication bias for the meta-analyses of mortality and the incidence of adverse effects. The mortality results indicate that there was no publication bias (P > 0.05) (Figure 23(a)); however, the publication bias identification of adverse reaction incidence implies that there may be publication bias (P < 0.05) (Figure 23(b)). Other outcomes of the publication bias did not occur since the number of included publications was less than 10.
Figure 23.

Publication bias: (a) mortality; (b) the rate of improvement of shortness of breath.
4. Discussion
Measures to treat infectious diseases with CP therapy have been developed for a long time. Many diseases, such as SARS [15], and the 2009 influenza A (H1N1) pandemic, have been well documented [37]. Previous studies have shown that the short-term mortality rate can be reduced in COVID-19 patients treated with severe respiratory failure with CP therapy [38]. However, it was recently reported in severe COVID-19 that CP therapy has nothing to do with clinical benefits [39]. The effectiveness of CP in the treatment of COVID-19 is controversial. In terms of size and test indicator coverage, the current meta-analysis is the largest RCT study meta-analyzed on the COVID-19 fatality research. These results provide evidence that CP therapy can reduce motility and, to some extent, improve clinical presentation. In this meta-analysis, 13 RCTs were included with a systematic review and meta-analysis to evaluate CP therapy in treating the novel coronavirus pneumonia. The analysis showed that most patients tolerated CP transfusions well and that CP therapy was safe. The CP group had a considerably reduced death risk than the control group. The CP group considerably decreased inflammatory indicators, including IL-6 and Ferritin. In terms of clinical manifestation, there was a clear improvement in the CP group's shortness of breath. These findings support the effectiveness of convalescent plasma as a COVID-19 treatment strategy.
As shown in our analysis, CP treatment could not increase SARS-CoV-2 clearance ability in COVID-19 patients but have benefits in reducing mortality. Reductions in inflammatory indicators such as chemokines, cytokines, IL-6, and ferritin protein may be linked to the process through which CP therapy lowers mortality. The neutrophils and lymphocytes in this investigation did not significantly differ between the two groups. CP therapy, however, resulted in significantly lower plasma levels of inflammatory cytokines than the control group. Those results indicated that the possible mechanism of CP treatment might be reducing inflammatory markers, improving gas exchange, reducing oxygen requirements, and improving shortness of breath. Those results prove the reduction in mortality observed in patients receiving CP treatment. This analysis was in accordance with a recent report [40]. In terms of days to hospital discharge and rate of removal from the ventilator, a study evaluating the safety of convalescent plasma therapy based on acute coronavirus, influenza, and Ebola virus infections found no benefit. This corresponds to the findings of a similar study [41].
Compared with other published literature on CP therapy for COVID-19, this meta-analysis has the advantage of involving the most randomized controlled trials. The 13 included articles were all randomized controlled trials of high quality, which did not selectively report the results, and had the most comprehensive outcome indicators. However, we admit that our research has several limitations. First, those included studies differ in size, risk of bias, and external validity. Second, given the limitations of database searches and manual retrieval, it is not certain that all published reports on CP treatment for COVID-19 have been included. Third, the differences between mild, moderate, severe, and critically ill patients were not evaluated, rendering it unclear whether the curative efficacy of CP therapy varied between different populations. Fourth, the follow-up period in all the included publications was insufficient to observe long-term consequences. We will continue to monitor and update the literature analysis as new evidence emerges.
5. Conclusions
The safety and potential efficacy of convalescent plasma therapy offer a promising treatment strategy for COVID-19. With no adverse effects, CP treatment can reduce mortality and improve breath and inflammatory cytokines IL-6 and Ferritin in COVID-19. It has little effect on improving virology indicators. As a result, additional high-quality clinical trials are required to validate these results.
Acknowledgments
This work was supported by the Fund project of the University of South China for Prevention and Control of COVID-19 (grant number 2020-22) and the Scientific Research Fund Project of Hunan Provincial Health Commission (grant number 20201983).
Contributor Information
Zhongtian Peng, Email: hncspzt@163.com.
Cai Yan, Email: yancaiisland@163.com.
Data Availability
All data generated or analyzed during this study are included in this article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Lu H., Stratton C. W., Tang Y. W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. Journal of Medical Virology . 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Oecologica . 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hossain M. J., Ahmmed F., Khan M. R., et al. Impact of prolonged COVID-19 lockdown on body mass index, eating habits, and physical activity of university students in Bangladesh: a web-basedcross-sectional study. Frontiers in Nutrition . 2022;9 doi: 10.3389/fnut.2022.873105.873105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coomes E. A., Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Reviews in Medical Virology . 2020;30(6):1–9. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison A. G., Lin T., Wang P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends in Immunology . 2020;41(12):1100–1115. doi: 10.1016/j.it.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahman M. M., Ahmed M., Islam M. T., et al. Nanotechnology-based approaches and investigational therapeutics against COVID-19. Current Pharmaceutical Design . 2022;28(12):948–968. doi: 10.2174/1381612827666210701150315. [DOI] [PubMed] [Google Scholar]
- 7.Rahman M. M., Islam M. R., Shohag S., et al. Multifaceted role of natural sources for COVID-19 pandemic as marine drugs. Environmental Science and Pollution Research . 2022;29(31):46527–46550. doi: 10.1007/s11356-022-20328-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Islam F., Bibi S., Meem A. F. K., et al. Natural bioactive molecules: an alternative approach to the treatment and control of COVID-19. International Journal of Molecular Sciences . 2021;22(23):p. 12638. doi: 10.3390/ijms222312638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phan T. Genetic diversity and evolution of SARS-CoV-2. Infection, Genetics and Evolution . 2020;81 doi: 10.1016/j.meegid.2020.104260.104260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matin M. M., Uzzaman M., Chowdhury S. A., Bhuiyan M. M. H. In vitro antimicrobial, physicochemical, pharmacokinetics and molecular docking studies of benzoyl uridine esters against SARS-CoV-2 main protease. Journal of Biomolecular Structure and Dynamics . 2022;40(8):3668–3680. doi: 10.1080/07391102.2020.1850358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Islam F., Dhawan M., Nafady M. H., et al. Understanding the omicron variant (B.1.1.529) of SARS-CoV-2: mutational impacts, concerns, and the possible solutions. Annals of Medicine and Surgery . 2022;78 doi: 10.1016/j.amsu.2022.103737.103737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiersinga W. J., Rhodes A., Cheng A. C., Peacock S. J., Prescott H. C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA . 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 13.Keller M. A., Stiehm E. R. Passive immunity in prevention and treatment of infectious diseases. Clinical Microbiology Reviews . 2000;13(4):602–614. doi: 10.1128/cmr.13.4.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levin M. J., Ustianowski A., De Wit S., et al. Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of covid-19. The New England Journal of Medicine . 2022;386(23):2188–2200. doi: 10.1056/nejmoa2116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soo Y. O., Cheng Y., Wong R., et al. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clinical Microbiology and Infection . 2004;10(7):676–678. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mair-Jenkins J., Saavedra-Campos M., Baillie J. K., et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. The Journal of Infectious Diseases . 2015;211(1):80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraft C. S., Hewlett A. L., Koepsell S., et al. The use of TKM-100802 and convalescent plasma in 2 patients with Ebola virus disease in the United States. Clinical Infectious Diseases . 2015;61(4):496–502. doi: 10.1093/cid/civ334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robbiani D. F., Gaebler C., Muecksch F., et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature . 2020;584(7821):437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rojas M., Rodriguez Y., Monsalve D. M., et al. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmunity Reviews . 2020;19(7) doi: 10.1016/j.autrev.2020.102554.102554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duan K., Liu B., Li C., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proceedings of the National Academy of Sciences of the United States of America . 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen C., Wang Z., Zhao F., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA . 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cumpston M., Li T., Page M. J., et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Library: Cochrane Reviews . 2019;10 doi: 10.1002/14651858.ED000142.ED000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page M. J., Moher D., Bossuyt P. M., et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ . 2021;372:p. n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salman O. H., Mohamed H. Efficacy and safety of transfusing plasma from COVID-19 survivors to COVID-19 victims with severe illness. A Double-Blinded Controlled Preliminary Study . 2020;36(1):264–272. [Google Scholar]
- 25.Ali S., Uddin S. M., Shalim E., et al. Hyperimmune anti-COVID-19 IVIG (C-IVIG) treatment in severe and critical COVID-19 patients: a phase I/II randomized control trial. E-Clinical Medicine . 2021;36 doi: 10.1016/j.eclinm.2021.100926.100926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.AlQahtani M., Abdulrahman A., Almadani A., et al. Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease. Scientific Reports . 2021;11(1):p. 9927. doi: 10.1038/s41598-021-89444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett-Guerrero E., Romeiser J. L., Talbot L. R., et al. Severe acute respiratory syndrome coronavirus 2 convalescent plasma versus standard plasma in coronavirus disease 2019 infected hospitalized patients in New York: a double-blind randomized trial. Critical Care Medicine . 2021;49(7):1015–1025. doi: 10.1097/CCM.0000000000005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gharbharan A., Jordans C. C. E., GeurtsvanKessel C., et al. Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection. Nature Communications . 2021;12(1):p. 3189. doi: 10.1038/s41467-021-23469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peter W. H., Lise E., Leon P., et al. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet . 2021;397(10289):2049–2059. doi: 10.1016/S0140-6736(21)00897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L. Correction to data in trial of convalescent plasma treatment for COVID-19. JAMA . 2020;324(5):p. 519. doi: 10.1001/jama.2020.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Libster R., Perez Marc G., Wappner D., et al. Early high-titer plasma therapy to prevent severe covid-19 in older adults. The New England Journal of Medicine . 2021;384(7):610–618. doi: 10.1056/nejmoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pouladzadeh M., Safdarian M., Eshghi P., et al. A randomized clinical trial evaluating the immunomodulatory effect of convalescent plasma on COVID-19-related cytokine storm. Internal and Emergency Medicine . 2021;16(8):2181–2191. doi: 10.1007/s11739-021-02734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Donnell M. R., Grinsztejn B., Cummings M. J., et al. A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19. Journal of Clinical Investigation . 2021;131(13) doi: 10.1172/JCI150646.150646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simonovich V. A., Burgos Pratx L. D., Scibona P., et al. A randomized trial of convalescent plasma in covid-19 severe pneumonia. The New England Journal of Medicine . 2021;384(7):619–629. doi: 10.1056/nejmoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarwal A., Mukherjee A., Kumar G., et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ . 2020;371 doi: 10.1136/bmj.m3939.m3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasheed A. M., Fatak D. F., Hashim H. A., et al. The therapeutic potential of convalescent plasma therapy on treating critically-ill COVID-19 patients residing in respiratory care units in hospitals in Baghdad, Iraq. InfezMed-Infezmed . 2020;28(3):357–366. [PubMed] [Google Scholar]
- 37.Hung I. F., To K. K., Lee C. K., et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clinical Infectious Diseases . 2011;52(4):447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perotti C., Baldanti F., Bruno R., et al. Mortality reduction in 46 severe Covid-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter trial. Haematologica . 2020;105(12):2834–2840. doi: 10.3324/haematol.2020.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Omrani A. S., Zaqout A., Baiou A., et al. Convalescent plasma for the treatment of patients with severe coronavirus disease 2019: a preliminary report. Journal of Medical Virology . 2021;93(3):1678–1686. doi: 10.1002/jmv.26537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghadami L., Hasibi M., Asadollahi-Amin A., Asanjarani B., Farahmand M., Abdollahi H. Convalescent plasma therapy in patients with severe COVID-19, a single-arm, retrospective study. Microbial Pathogenesis . 2022;165 doi: 10.1016/j.micpath.2022.105482.105482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Devasenapathy N., Ye Z., Loeb M., et al. Efficacy and safety of convalescent plasma for severe COVID-19 based on evidence in other severe respiratory viral infections: a systematic review and meta-analysis. CMAJ . 2020;192(27):E745–E755. doi: 10.1503/cmaj.200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.


