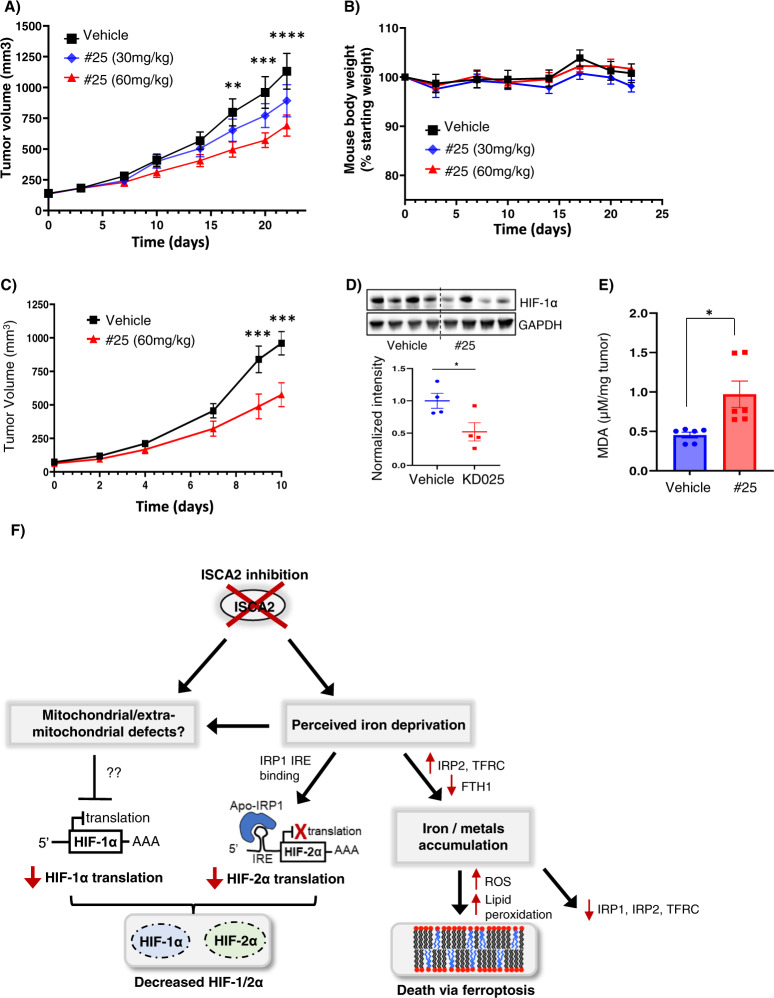
Fig. 8. ISCA2 inhibition inhibits xenograft growth in vivo.
A, B Effect of treatment of 786-0 subcutaneous xenografts with vehicle or indicated doses of compound #25 (8 mice/group) administered orally once daily, on tumor volume (A), or mouse body weights (B). **p < 0.01; ***p < 0.001; ****p < 0.0001 from Students’ T-tests of vehicle versus 60 mg/kg treated group. p was not significant (NS) for 30 mg/kg treated group versus vehicle. C Effect of treatment of RENCA subcutaneous xenografts in syngeneic Balb/c mice with vehicle or 60 mg/kg #25 (8 mice/group) once daily. D Western blots showing HIF-1α and GAPDH with densitometric values normalized to GAPDH below. E MDA content of RENCA tumors from mice treated with vehicle or 60 mg/kg #25. F Proposed model for effects of ISCA2 blockade. Inhibition of ISCA2 using either small molecules or siRNA results in perceived iron deprivation, which induces upregulation of IRP2, TFRC and downregulation of FTH1, that together promote iron/metals accumulation that triggers cell death via ferroptosis, and also drives the later downregulation of IRP1, IRP2 and TFRC. This perceived iron deprivation also drives a shift to the IRE-binding form of IRP1 which inhibits HIF-2α translation. ISCA2 inhibition may also cause other mitochondrial/extramitochondrial defects dependent or independent of perceived iron deprivation that inhibits the translation of HIF-1α through unknown mechanisms. Thus, ISCA2 inhibition depletes both HIF-1α and HIF-2α and promotes cell death via ferroptosis.