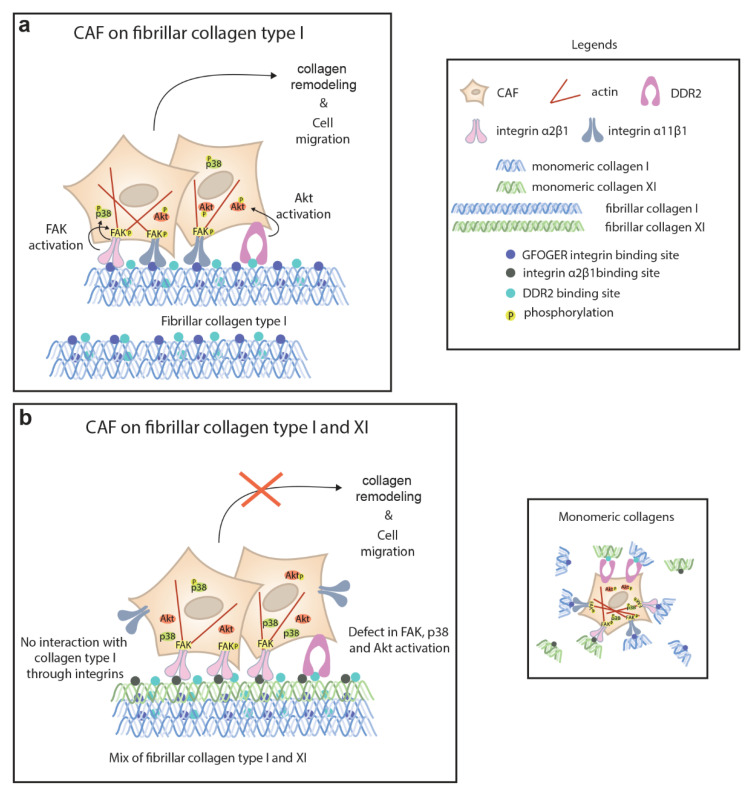
Figure 5.
In collagen type I matrices, cancer-associated fibroblasts (CAFs) interact with fibrillar collagen through α2β1 and α11β1 integrins and DDR2 leading to the activation of FAK, p38 and Akt, which in turn promote collagen remodeling and cell migration. (a) Both α2β1 and α11β1 integrins recognize the GFOGER motif present in collagen type I. Integrins, via their link to actin cytoskeleton, control cell contractility and motility, whereas DDR2 could modulate integrin activity. In the presence of collagen type XI in collagen type I fibrils, integrin-binding sites on collagen type I are not available, inhibiting integrin α11β1 function. (b) Although integrin α2β1 and DDR2 can interact with collagen type XI, there is a defect of FAK and Akt long-term activation, resulting in inhibition of collagen remodeling and cell migration. On monomeric collagen, single molecules of collagen do not interfere between each other.