Figure 3.
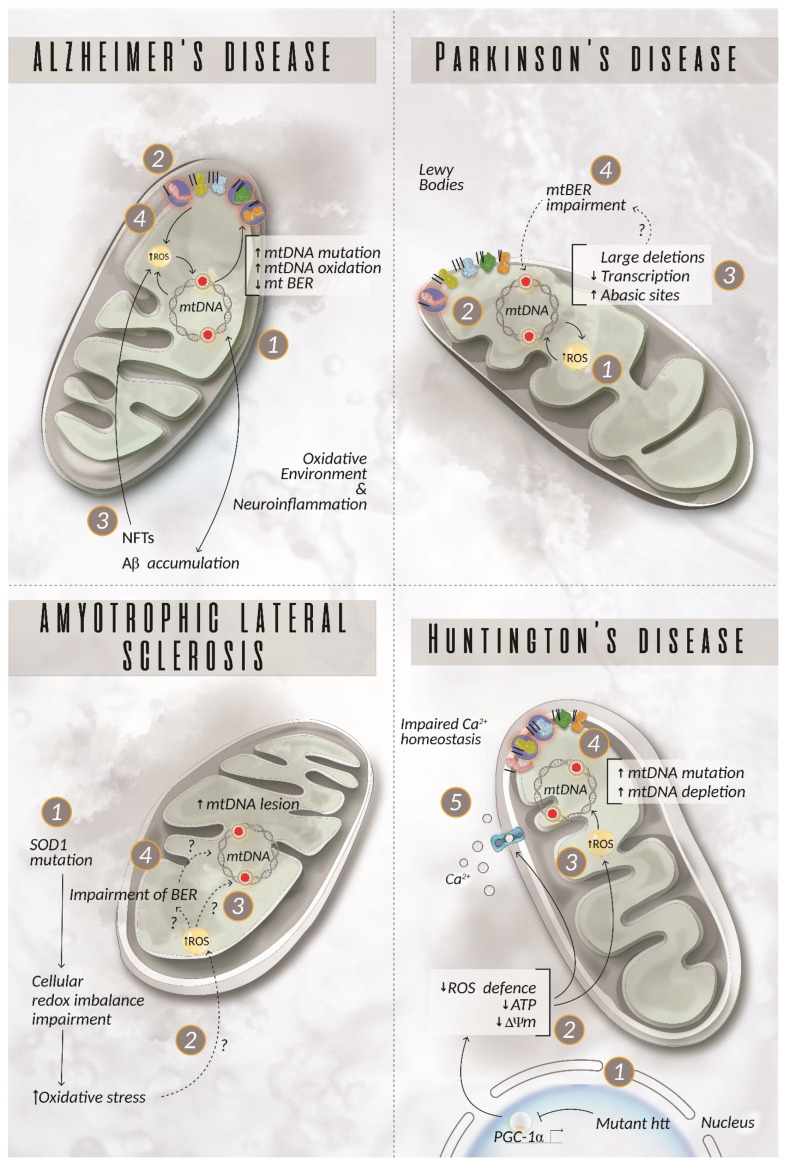
mtDNA damage in neurodegenerative diseases. Alzheimer’s disease (AD). mtDNA damage in AD can lead to energy failure (1) driven by the defective complexes I, III, IV, and V (2), promotion of Aβ accumulation (3) and increased oxidative stress (4), which, in turn, exacerbates mtDNA damage and increased production of ROS, creating a vicious cycle of dysfunctional and damaged mitochondria. Parkinson’s disease (PD). An incremented production of ROS (1) increases the susceptibility of mtDNA to damage (2). PD patients display either an increased amount of abasic sites and/or large mtDNA deletions (3) with a consequent failure in the formation of a fully functional OXPHOS system. Up to now, there have been no clear explanations about the mechanisms underpinning the dysfunction detected in the mitochondria in patient neurons, but the involvement of defects at the level of the mitochondrial BER is plausible (4). Amyotrophic lateral sclerosis (ALS). Mutations in SOD1 cause a cellular redox imbalance (1), but it is not clear how this phenomenon affects mtDNA stability. The most recent theory suggests an indirect role of mutated SOD1 on the proteins involved in mtDNA repair rather than a direct effect on ROS production with consequent mtDNA damage (2 and 3) [124]. As of yet, there is no evidence for this hypothesis even though the alteration of the nDNA repair system supports the impairment of the mitochondrial BER as documented in ALS (4) [125,126,127]. Huntington’s disease (HD). Mutant huntingtin (htt) has indirect toxic effects on mtDNA. It suppresses the expression of PGC-1α (1), which negatively impacts ROS scavenging mechanisms, ATP production, mitochondrial membrane potential, and, more generally, the whole mitochondrial physiology (2). Correspondingly, ROS production is exacerbated (3), leading to increased mtDNA mutation and depletion, consequently disrupting mitochondrial integrity (4). Studies have also underlined the presence of imbalanced Ca2+ homeostasis in HD patients (5). Highlighted complexes with a circle represent components of the OXPHOS system, in which a mutation related to the disease described has been reported. Aβ = amyloid beta peptide; BER = base excision repair; NFTs = neurofibrillary tangles; ROS = reactive oxygen species; SOD1 = superoxide dismutase 1.