Figure 1.
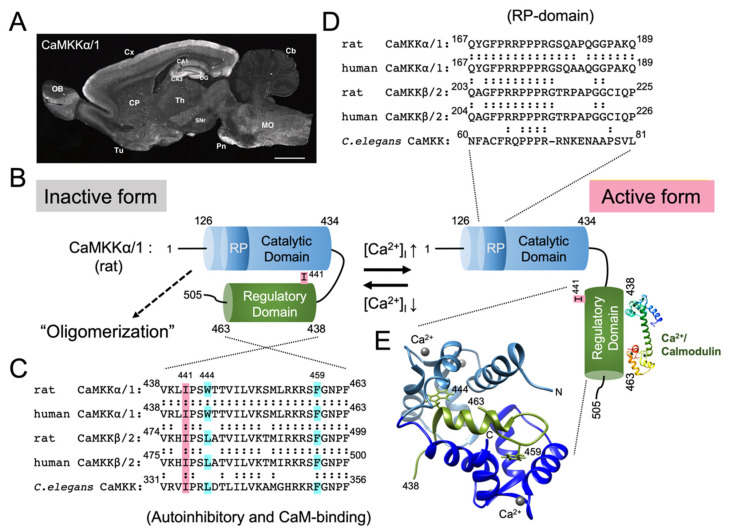
CaMKKα/1; activation mechanism, immunohistochemical localization in the rat brain, and Ca2+/CaM-binding. (A) Sagittal section of the adult rat brain immunostained with a monoclonal antibody against CaMKKα/1 (reproduced from Ref. [61], with permission from John Wiley and Sons). CA1 and CA3, CA1 and CA3 subregions of Ammon’s horn of the hippocampus; Cb, cerebellar cortex; CP, caudate putamen; Cx, cerebral cortex; DG, dentate gyrus; MO, medulla oblongata; OB, olfactory bulb; Pn, pontine nuclei; SNr, substantia nigra pars reticulata; Th, thalamus; and Tu, olfactory tubercle. Scale bar = 2.5 mm. (B) Proposed model of CaMKKα/1 activation mechanism. At low intracellular Ca2+ concentration, CaMKKα/1 is in an inactive conformation, where the catalytic domain (residues 126−434) is tightly associated with the regulatory domain (residues 438−463, C). With increasing intracellular Ca2+ concentration, Ca2+/ CaM binds to regulatory domain of CaMKKα/1 (E) to suppress autoinhibition, thereby activating the kinase [64]. An activated CaMKK recognizes and phosphorylates downstream kinases including CaMKI, IV, and AMPK by using an Arg/Pro rich insert domain (RP-domain, D) [39,65]. Amino acid sequence alignments of the regulatory domain including the autoinhibitory and Ca2+/CaM binding segments (C) and RP-domain (D) in various CaMKKs (rat, human α/1 and β/2 isoforms, and C. elegans). Trp(W)444 and Phe(F)459 in rat CaMKKα/1 (C) are conserved anchoring residues (indicated by light blue boxes) to the N- and C-terminal hydrophobic pockets of Ca2+/CaM, respectively [66]. Ile(I)441 (indicated by a pink box, C) is important for rat CaMKKα/1 autoinhibition [64]. (E) Ribbon diagram of the NMR structure of Ca2+/CaM-CaMKKα/1 regulatory domain peptide (residues 438−463, C) complex was obtained from the Protein Data Bank (PDB) entry 1ckk [66] and was visualized using the UCF Chimera [67]. Modified from Ref. [68].