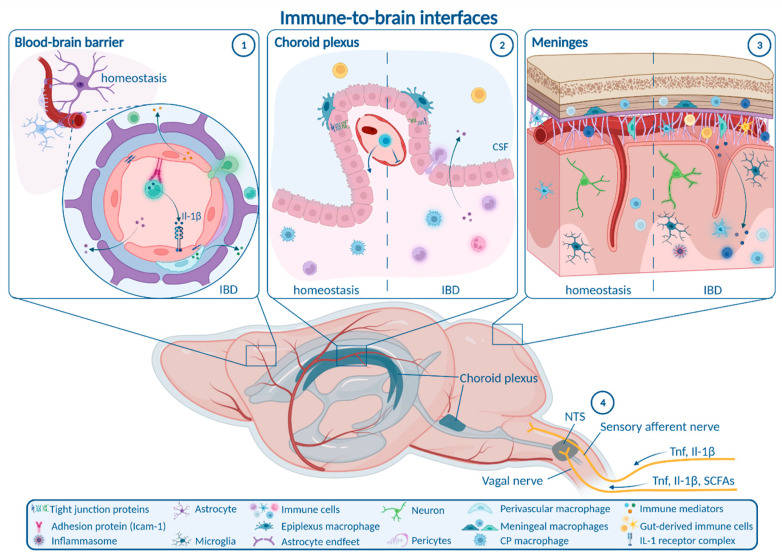
Figure 1.
Alterations at immune-to-brain interfaces during IBD. (1) At the blood–brain barrier (BBB), downregulation of tight junction proteins and endothelial permeability mediate influx of inflammatory molecules and immune cells. Additional non-disruptive BBB changes comprise endothelial upregulation of adhesion proteins for the interaction and transmigration of circulating immune cells, as well as the release of mediators by endothelial cells and perivascular macrophages that modulate microglial and neuronal function. (2) At the choroid plexus (CP), decreased expression of tight junction proteins and increased fenestration followed by transient closure of the vascular barrier are observed, whereas permeability of the epithelial barrier is transiently enhanced. Numbers of stromal monocytes and neutrophils are increased during intestinal inflammation. (3) In the meninges, infiltration of gut-derived immune cells as well as activation of meningeal immune cells and the NLRP3 inflammasome can be detected. (4) Neural communication pathways linking gut inflammation and the brain include the afferent input via the vagal nerve to the nucleus tractus solitarii (NTS), and information from sensory afferent neurons stimulated in the periphery that provoke neural activation in different brain regions. Gut-derived immune cells (2) can enter the CSF via the CP or the meninges. CP: choroid plexus; CSF: cerebrospinal fluid; SCFA: short chain fatty acids; Tnf: tumor necrosis factor; Il-1β: interleukin-1β; NTS: nucleus tractus solitarii. Figure created with BioRender.com (accessed on 15 September 2022).