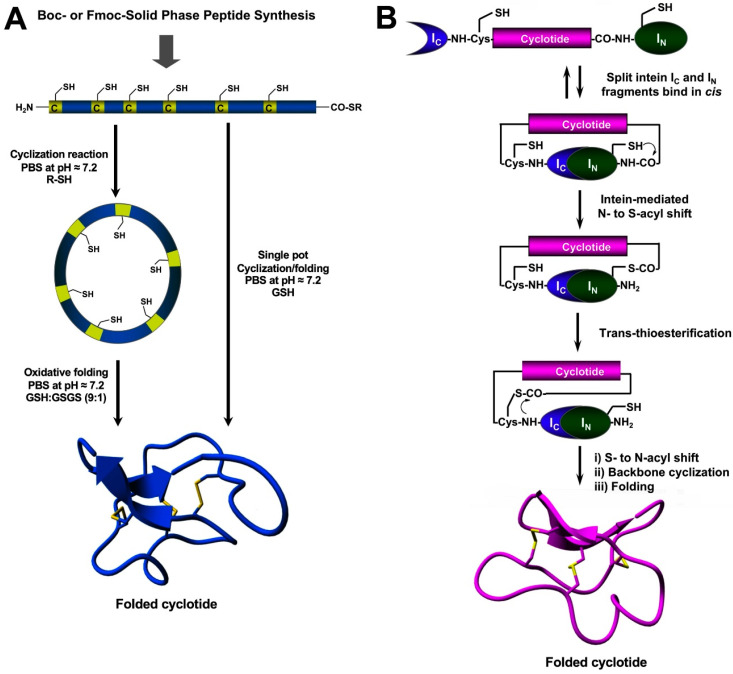
Figure 4.
Different methods for the generation of native-folded cyclotides. (A) Chemical synthesis of cyclotides by employing an intramolecular version of native chemical ligation. This method requires the chemical production of a linear cyclotide precursor containing both an α-thioester moiety at the C-terminus and an N-terminal Cys residue. The linear precursor is then cyclized under reductive conditions and finally oxidatively folded using a proper redox buffer. The cyclization and oxidative folding reactions can be also efficiently performed in a ‘single pot’ reaction. This is accomplished by performing the cyclization in the presence of reduced GSH as the thiol cofactor. (B) Heterologous expression of cyclotides can be accomplished using protein trans-splicing (PTS) This approach has been employed for the generation of several MCoTI-cyclotides by using the native Cys residue located at the N-terminus of loop 6 to facilitate backbone cyclization. This method has been used to produce bioactive cyclotides using either eukaryotic or prokaryotic expression systems. Figure adapted from reference [23].