Table 1.
Agonist properties of compounds Norbo 1–28 at the 5-HT1A receptor.

| ||||
|---|---|---|---|---|
| 5-HT1A Receptor G-Protein Stimulation | ||||
| Compd | X | pEC50 | EC50 (95% CI, nM) | Emax (%) ± SEM |
|
Norbo-1 (exo) |

|
5.92 ± 0.09 | 1200 (778–1850) | 174 ± 4.15 * |
|
Norbo-2 (endo) |

|
5.78 ± 0.08 | 1633 (1111–2400) | 188 ± 4.9 ** |
|
Norbo-3 (exo) |
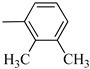
|
6.75 ± 0.1 | 177 (110–285) && | 185 ± 3.2 *** |
|
Norbo-4 (endo) |

|
6.74 ± 0.08 | 181 (123–266) &&& | 181 ± 2.4 *** |
|
Norbo-5 (exo) |

|
5.0 ± 0.49 | 96,070 (9001–102,500) & | 140 ± 25.2 && |
|
Norbo-6
endo |

|
5.5 ± 0.12 | 3218 (1228–8435) | 131 ± 5.5 && |
|
Norbo-7 (exo) |

|
6.46 ± 0.12 | 348 (191–632) && | 169 ± 3.5 * |
|
Norbo-8 (endo) |

|
6.78 ± 0.09 | 165 (107–253) &&& | 167 ± 2.3 * |
|
Norbo-9 (exo) |

|
6.68 ± 0.16 | 207 (95–450) & | 149 ± 3.1 |
|
Norbo-10 (endo) |

|
6.7 ± 0.1 | 198 (117–333) &&& | 163 ± 2.4 # |
|
Norbo-11 (exo) |

|
no activity | no activity | no activity |
|
Norbo-12 (endo) |

|
5.7 ± 0.25 | 1875 (549–6407) # | 127 ± 5.1 ###,&&& |
|
Norbo-13 (exo) |

|
no activity | no activity | no activity |
|
Norbo-14 (endo) |
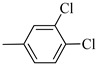
|
no activity | no activity | no activity |
|
Norbo-15 (exo) |

|
6.0 ± 0.13 | 926 (503–1706) | 161 ± 4.5 |
|
Norbo-16 (endo) |

|
6.0 ± 0.11 | 939 (533–1654) | 182 ± 5.5 **,# |
|
Norbo-17 (exo) |

|
6.2 ± 0.17 | 553 (242–1264) | 122 ± 2.0 &&& |
|
Norbo-18 (endo) |

|
5.6 ± 0.15 | 2321 (1132–4761) | 143 ± 5.1 &&& |
|
Norbo-19 (exo) |
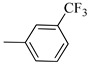
|
6.5 ± 0.08 | 283 (1880–4276) && | 162 ± 2.1 * |
|
Norbo-20 (endo) |

|
6.7 ± 0.05 | 195 (1483–2559) &&& | 167 ± 1.4 ** |
|
Norbo-21 (exo) |
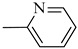
|
6.2 ± 0.06 | 588 (433–798) | 181 ± 2.5 ** |
|
Norbo-22 (endo) |
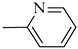
|
6.1 ± 0.09 | 782 (513–1192) | 186 ± 4.4 *** |
|
Norbo-23 (exo) |

|
5.62 ± 0.1 | 2398 (1143–3567) | 181 ± 4.1 ** |
|
Norbo-24 (endo) |

|
5.76 ± 0.1 | 1720 (1049–2819) | 177 ± 6.1 * |
|
Norbo-25 (exo) |

|
5.7 ± 0.1 | 1911 (1156–3159) # | 184 ± 6.4 ** |
|
Norbo-26 (endo) |

|
5.1 ± 0.1 | 7502 (2448–23000) | 178 ± 22.6 * |
|
Norbo-27 (exo) |

|
no activity | no activity | no activity |
|
Norbo-28 (endo) |

|
no activity | no activity | no activity |
| 8-OH-DPAT | 7.5 ± 0.11 | 27.2 (18–52) | 154 ± 2.3 | |
One-way ANOVA, Bonferroni post-hoc test. Statistical significance was marked as follows: * NORBO vs. 8-OH-DPAT; # exo isomer vs. endo isomer; & vs. corresponding 4-phenylpiperazin-1-yl substituted isomer. One, two or three symbols represent significance levels of 0.05, 0.01, and 0.001, respectively. EC50—half-maximal effective concentration (potency), Emax—efficiency.
