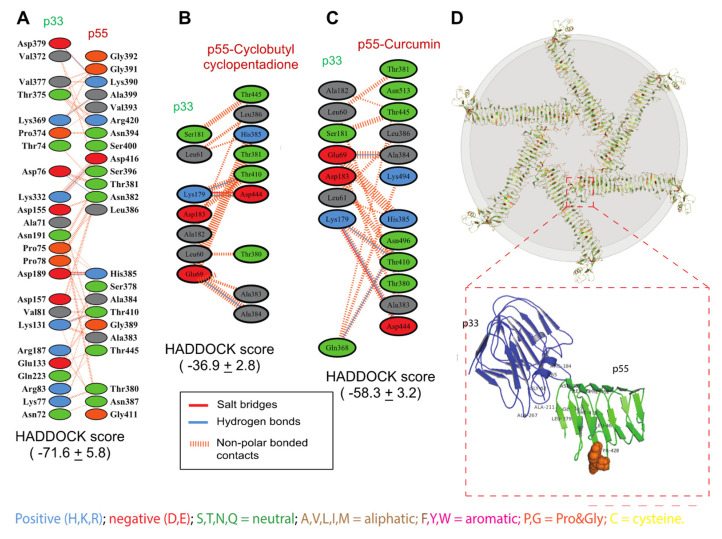
Figure 5.
Effect of Cyclobutyl cyclopentadine and Curcumin on p33 and p55 docking interaction and vacA protein hexamer. (A) Schematic representation of binding interaction between the p33 and p55 domains of VacA protein. Amino acid residues involved in the interaction between p33 and p55 domains are shown in different colors based on their chemical structure. Different bonding interactions between amino acids are shown in different colors i.e., salt bridges, hydrogen bonds, etc. (B) Schematic representation of binding interaction between the p33 and p55-Cyclobutyl cyclopentadione complex. Amino acid residues involved in the interaction between p33 and p55-Cyclobutyl cyclopentadione complex are shown in different colors based on their chemical structure. Different bonding interactions between amino acids are shown in different colors i.e., salt bridges, hydrogen bonds, etc. (C) Schematic representation of binding interaction between the p33 and p55-curcumin complex. Amino acid residues involved in the interaction between p33 and p55-curcumin complex are shown in different colors based on their chemical structure. Different bonding interactions between amino acids are shown in different colors i.e., salt bridges, hydrogen bonds, etc. (D) A blueprint of VacA protein hexamer formed by VacA protomers. p33 (blue colored) and p55 (green colored) domains involved in hexamer formation.