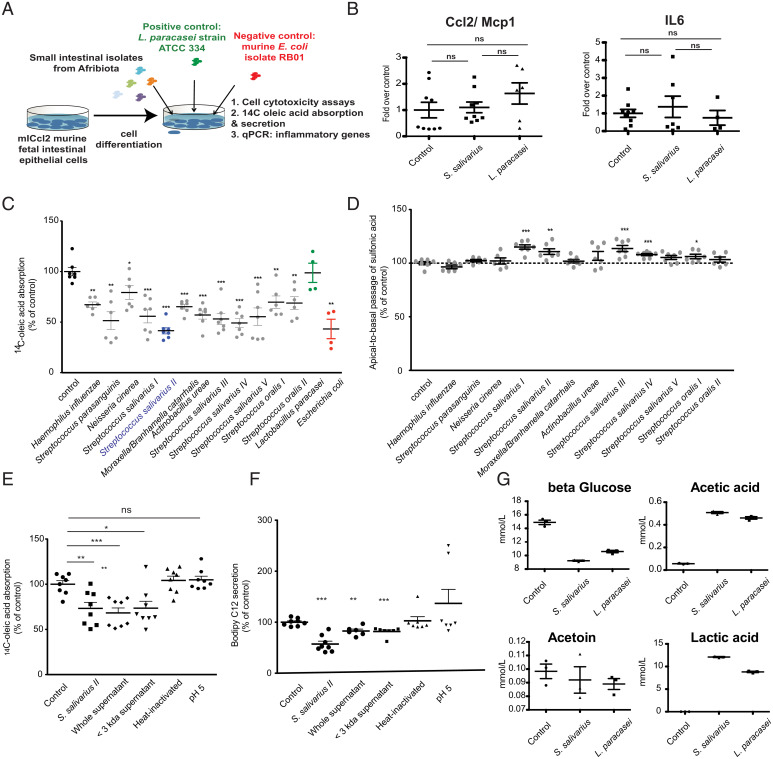
Fig. 4.
Duodenal isolates of oropharyngeal bacteria lead to decreased lipid absorption in vitro. (A) Experimental setup of the in vitro cell assay. (B) Expression of proinflammatory genes in m-IcCl2 cells overexposed to either a clinical, small intestinal isolate of S. salivarius or L. paracasei (control bacterium). Values are normalized to the geometric mean of the three housekeeping genes tbp, b2m, and gapdh. (C) Oleic acid absorption in polarized murine small intestinal cells (m-IcCl2) cocultured overnight with different clinical isolates from the duodenum of stunted children. (D) The sulfonic acid apical to basal permeability assay in polarized m-IcCl2 cells cocultured overnight with different clinical isolates from the duodenum of stunted children. (E) Oleic acid absorption in the context of live S. salivarius strain II, whole or filtered culture supernatant, or heat-killed cells of S. salivarius II or in a medium acidified to pH 5. (F) BODIPY C12 (lauric acid) secretion in the context of live S. salivarius strain II, whole or filtered culture supernatant, or heat-killed cells of S. salivarius II or in a medium acidified to pH 5. (G) NMR-quantified levels of metabolites in the supernatant of polarized m-IcCl2 cells cocultured for 16 h with control medium, S. salivarius, or L. paracasei. Experiments were performed in three independent replicates. Groups are compared using the Mann–Whitney U test. ns, P > 0.05. *P < 0.05; **P < 0.01; ***P < 0.001.