Figure 2.
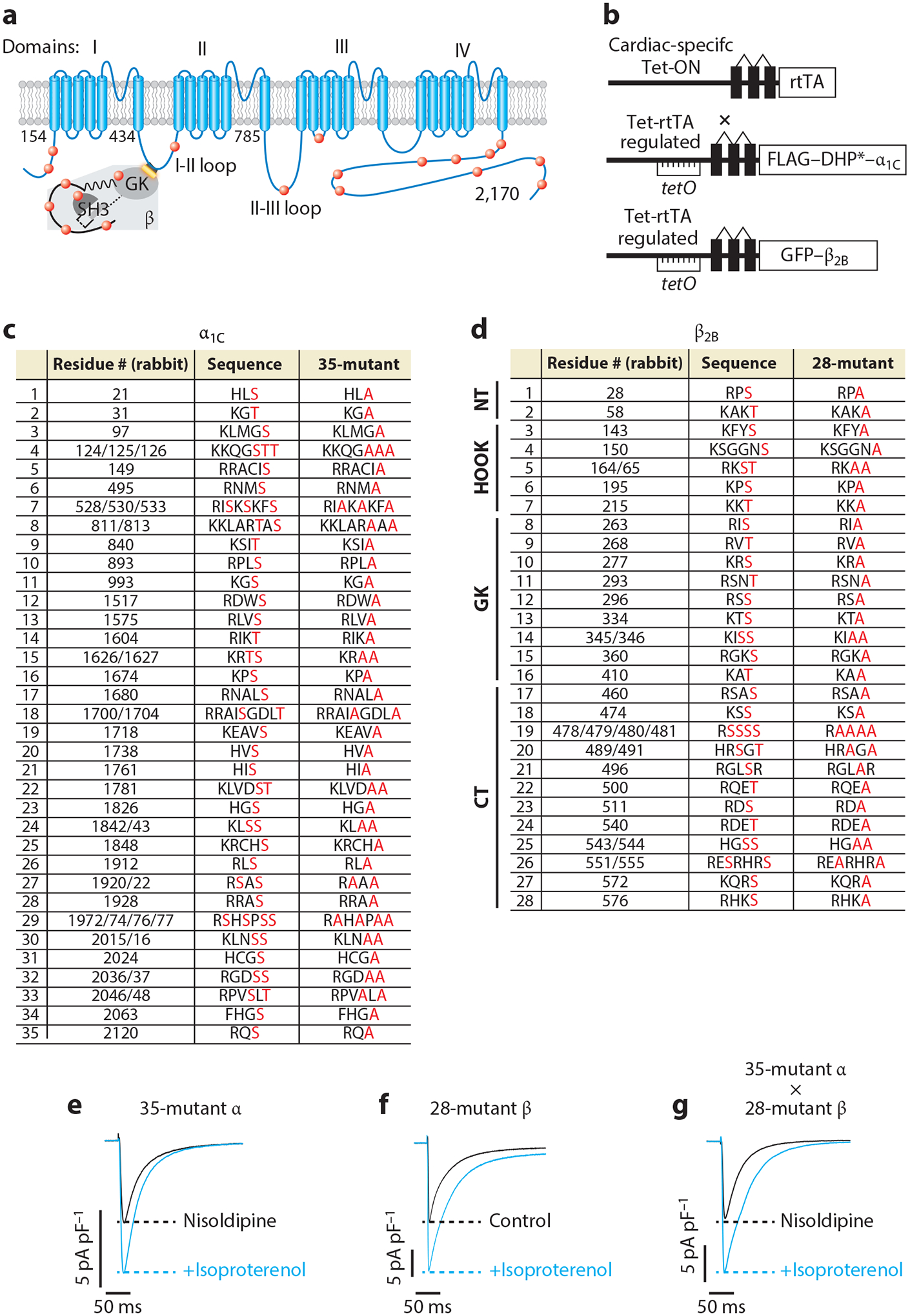
β-Adrenergic regulation of CaV1.2 does not require protein kinase A (PKA) phosphorylation of α1C and β subunits. (a) Schematic of rabbit cardiac α1C and β subunits. Red dots indicate some of the putative PKA phosphorylation sites. (b) Schematic of the transgene system (147) used for the creation of transgenic mice. Reverse tetracycline-controlled transactivator (rtTA) expression is driven by an α-myosin heavy chain (αMHC) promoter. The cDNAs for FLAG-DHP-resistant (DHP*) α1C or green fluorescent protein (GFP)-β2B were ligated behind tetO sequences. (c) The 35 putative PKA phosphorylation sites in rabbit α1C. The 51 residues in red, denoting predicted phosphorylation sites or within the immediate region of the predicted phosphorylation site, were replaced with alanine in the 35-mutant α1C transgenic mice. (d) The 37 residues within 28 sites are either predicted phosphorylation sites or within the immediate vicinity of predicted phosphorylation sites and were mutated to alanine in the 28-mutant β2B transgenic mice. The sites are distributed in the N-terminal (NT), HOOK, guanylate kinase–like (GK), and C-terminal (CT) domains of β2B. (e–g) Exemplar whole-cell CaV1.2 currents of 35-mutant α cardiomyocytes, 28-mutant β transgenic mice cardiomyocytes, and 35-mutant α X 28-mutant β transgenic mice cardiomyocytes. Figure adapted from Reference 68.
