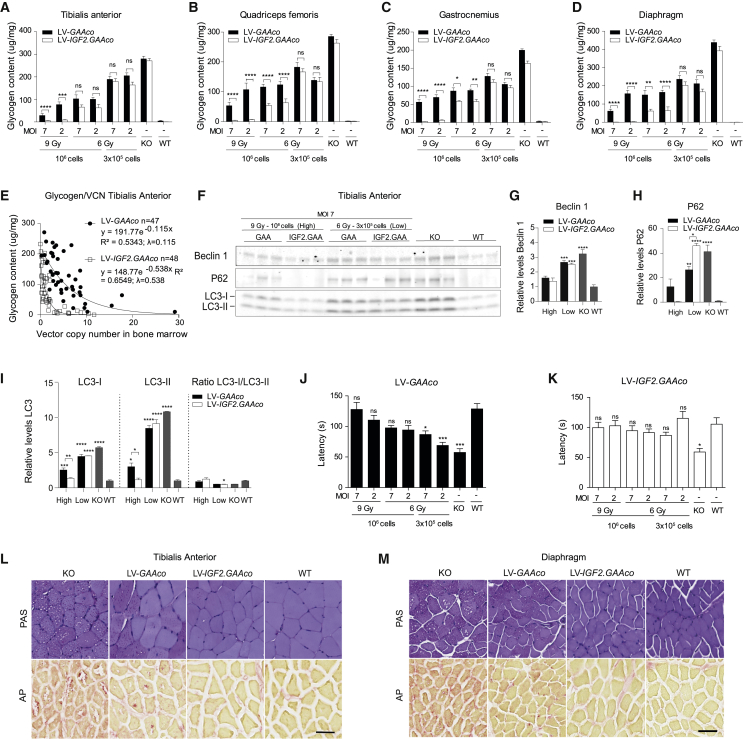
Figure 3.
Gene therapy with LV-IGF2.GAAco results in correction in skeletal muscles
(A–D) Total glycogen content in skeletal muscles after different doses of gene therapy with LV-IGF2.GAAco or LV-GAAco. MOI, multiplicity of infection; Gy, gray. (E) Correlation between bone marrow VCN and glycogen clearance in tibialis anterior. λ, exponential decay constant. VCN is not normalized for chimerism. (F–I) Immunoblot analysis in biological triplicates of tibialis anterior of mice treated with a high (MOI 7, 9 Gy, 106 cells) or low dose (MOI 7, 6 Gy, 3 × 105 cells) of gene therapy using antibodies against beclin 1, p62, and LC3. Density levels of beclin 1 (G), p62 (H), or LC3 (I) are quantified from (F) (loading controls are shown in Figure S5A). In (G)–(I), values are relative to WT. (J and K) Latency on a rotarod after gene therapy with LV-GAAco or (J) LV-IGF2.GAAco (K). (L and M) Representative images of PAS (upper row) and AP (lower row) stainings in tibialis anterior (L) and diaphragm (M) after high-dose gene therapy. Scale bar, 50 μm. Data are presented as means ± SEM. In (A)–(D), data are analyzed by two-way ANOVA with Bonferroni’s correction, using vector (LV-GAAco or LV-IGF2.GAAco) and gene therapy dose as categorical variables. Significant results are indicated by brackets. Results of multiple comparison analysis are reported in Table S3. In (G)–(K), data are analyzed by one-way ANOVA followed by Bonferroni’s multiple testing. Significance is expressed as relative to WT; other significant comparisons are indicated by brackets. (A–D; J, K) LV-GAAco and LV-IGF2.GAAco, n = 7; KO, n = 5; WT, n = 5. (G–I) n = 3 for all groups. (L and M) LV-GAAco and LV-IGF2.GAAco, n = 3 per group; KO, n = 2; WT, n = 2. ns, not significant; ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0 .001, ∗∗∗∗p ≤ 0 .0001.