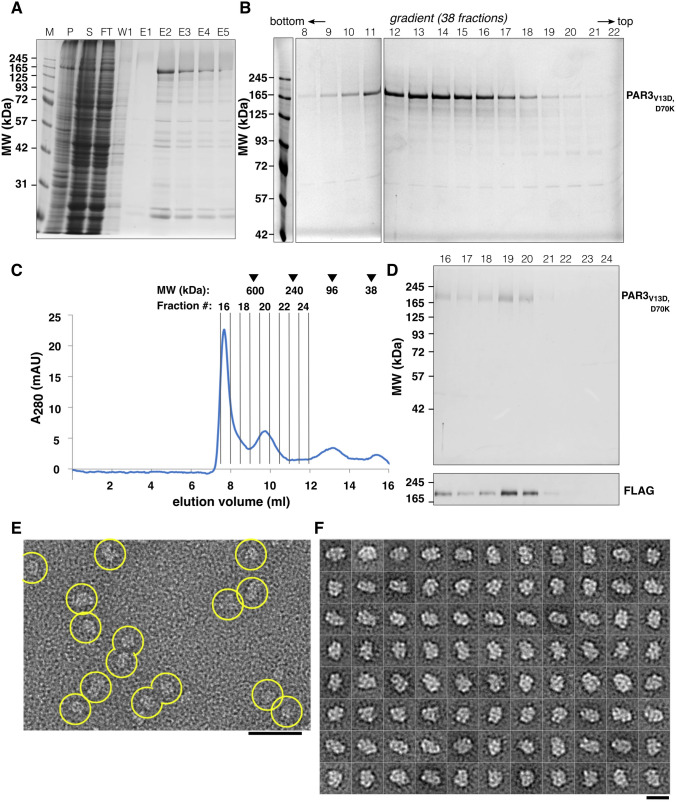
Fig. 3.
PAR3V13D,D70K is stable in solution and forms elongated particles. A Affinity selection of N-terminal 3 × FLAG-tagged PAR3V13D,D70K visualized by Coomassie-stained SDS-PAGE (M, marker; P, pellet; S, supernatant; FT, flow through; W1, wash fraction 1; E1-E5, elution fraction 1–5). B 5–20% glycerol gradient fractionation of PAR3V13D,D70K as visualized by Coomassie-stained SDS-PAGE. Shown are fractions 8–22 out of 38 fractions in total. PAR3V13D,D70K forms a defined peak on the gradient. C In SEC, PAR3V13D,D70K peaks in fractions 19 and 20. Position of calibration proteins (in kDa) and fractions used for SDS-PAGE analysis (D) are indicated at the top. D SEC fractions as indicated in C are separated by SDS-PAGE and visualized by Coomassie staining (top). The presence of PAR3V13D,D70K was confirmed by anti-FLAG western blot (bottom). E Single particles observed by negative stain EM. F Representative 2D class averages of PAR3V13D,D70K showing particles up to 20 nm in diameter. The scale bars correspond to 50 nm E and 20 nm F, respectively