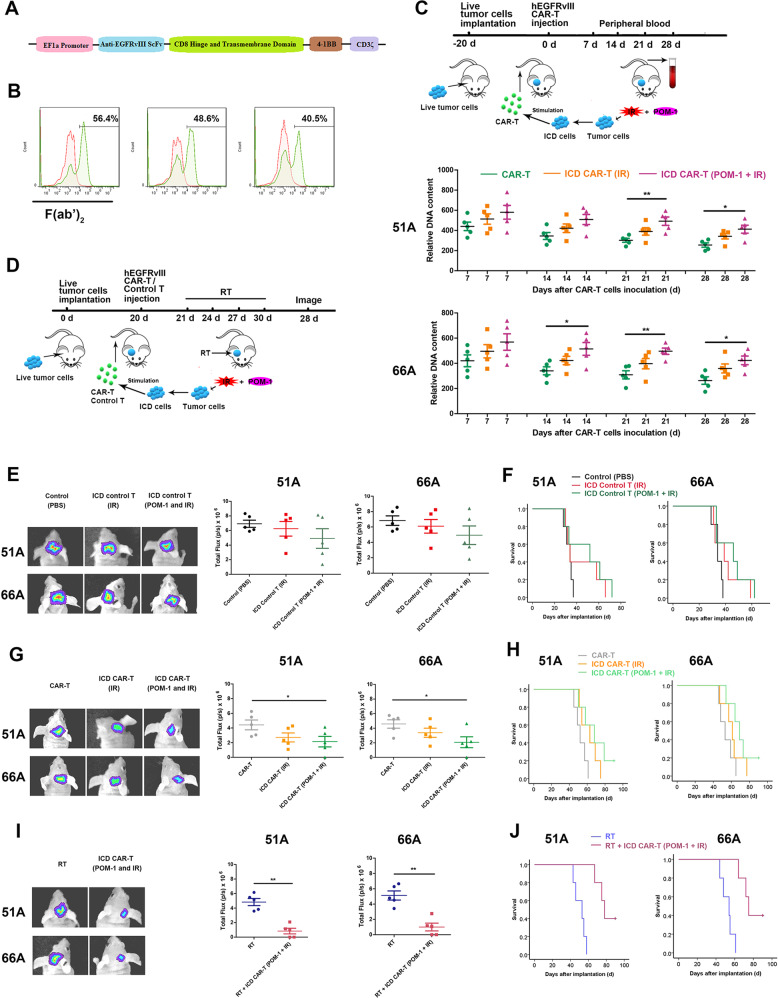
Fig. 6. ICD-stimulating hEGFRvIII CAR-T cells improve efficiently antitumor efficacy.
A The construction of EGFRvIII CARs was showed. B Expressions of CARs were confirmed by flow cytometry with F(ab’)2 antibody for human EGFRvIII. C ICD-activated hEGFRvIII CAR-T cells or CAR-T-cells were infused into the tail vein of nude mice with 51 A or 66 A xenografts. Peripheral blood was collected, and human genomic DNA in blood was detected using qPCR at an indicated time instead of CAR-T cells persistence in mice. n = 5. D Nude mice bearing tumor were injected with untransfected T cells or hEGFRvIII CAR-T cells with or without RT. The groups are following: 1. Control (PBS) group, the mice were injected with 100 μl PBS; 2. ICD Control T (IR) group, 51 A or 66 A cells were irradiated with 10 Gy and cultured for 4 days, then co-cultured with DCs for 24 hours. Untransfected T cells from healthy human PBMCs were added into culture system for 6 days. The activated T cells were administrated into mice via the tail vein on day 20 after 51 A or 66 A cells implantation; 3. ICD Control T (POM-1 and IR) group, GSCs were pretreated with POM-1 and irradiated, then treated as group 2; 4. CAR-T group, the mice were injected with hEGFRvIII CAR-Ts; 5. ICD CAR-T (IR) group, the hEGFRvIII CAR-Ts were treated as group 2; 6. ICD CAR-T (POM-1 and IR) group, the hEGFRvIII CAR-Ts were treated as group 3; 7. RT group, the mice were treated with RT 2 Gy once every 3 days for four times from day 21 after EGFRvIII transfected GSCs implantation. 8. RT plus ICD CAR-T (POM-1 and IR) group, the mice were treated with a combination of RT and hEGFRvIII CAR-Ts as group 6. Tumor size was measured by bioluminescence imaging signal (E) and the survival of mice was analyzed using Kaplan–Meier survival curves (F) after infusion of T cells from PBMCs. Tumor size (G) and survival (H) were evaluated after CAR-T infusion. The mice were treated by conventional RT after PBS or CAR-T injection, then tumor size (I) and survival (J) were evaluated after RT. n = 5. *P < 0.05, **P < 0.01.