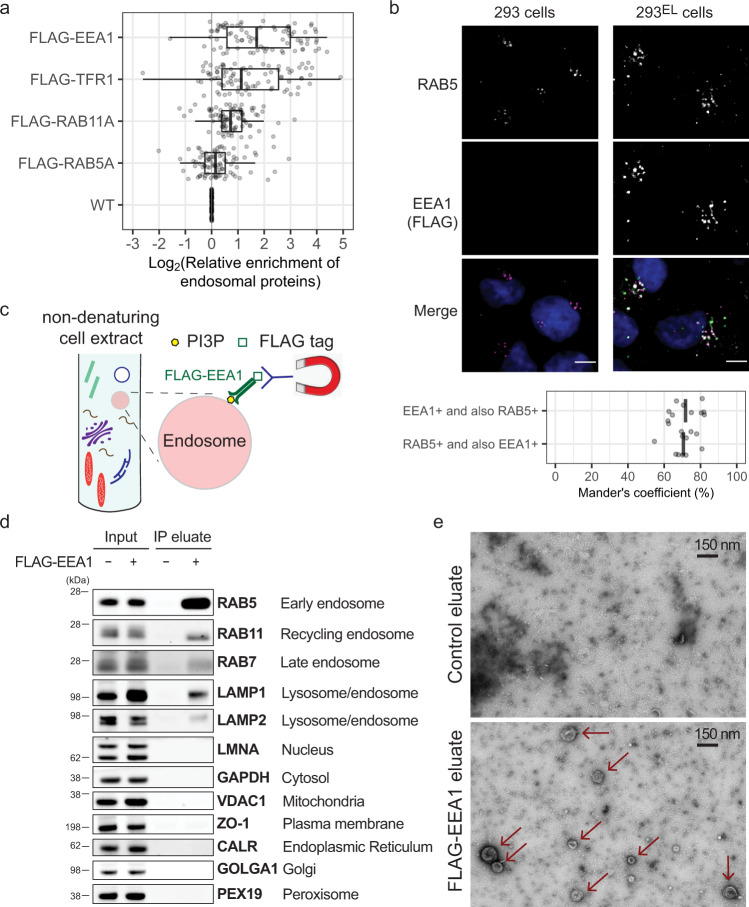
Fig. 1. Identification of EEA1 as a candidate affinity reagent for early endosome purification.
a The indicated FLAG-tagged proteins were stably expressed in 293L cells and non-detergent extracts subjected to immunoprecipitation with α-FLAG antibodies prior to proteomic analysis of trypsinized peptides using 10-plex TMT-based proteomics which included one replicate each of immunoprecipitates and whole-cell extracts in the 10-plex. The enrichment (Log2FC) of endosomal proteins32 (gray circles) relative to control 293L cells is shown, with FLAG-EEA1 displaying the greatest enrichment of proteins known to localize to endosomes. Left border, interior line, and right border in the box plot represent the 1st quartile, median, and 3rd quartile, respectively. b 293EL cells (clone 33) were subjected with immunofluorescence using α-FLAG (green, to detect FLAG-EEA1) and α-RAB5 (magenta) antibodies, and nuclei were stained with Hoechst 33342 (blue). Image analysis of 11 cells indicates that the Mander’s coefficient is ~0.7 for both the overlap of FLAG puncta with RAB5 and RAB5 puncta with FLAG. c Scheme depicting affinity purification of PI3P-positive early endosomes using FLAG-EEA1 in conjunction with α-FLAG antibodies immobilized on magnetic beads. d Control 293 cells or 293EL cells (clone 33) were lysed in non-denaturing buffer prior to either direct analysis of extracts by immunoblotting or were subjected to α-FLAG magnetic bead capture followed by immunoblotting with the indicated antibodies. Loaded amounts are equivalent to 0.06% and 6% of the input and IP eluate, respectively. e α-FLAG immunoprecipitates from either control 293 cells or 293EL cells (clone 33) were released from the affinity resin by FLAG peptide and then analyzed by transmission EM. Arrowheads indicate vesicular structures present in cells expressing FLAG-EEA1 but not control cells. Scale bar, 150 nm.