Abstract
The objective of this research was to investigate the effects of Mahuang and Tuer chickens, 2 representatives of the native chicken breed, and the slaughter age on meat quality and flavor compounds of soft-boiled chickens (SCs) in comparison to a commercial cross boiler. A total of 432 chicks were randomly allocated into the following groups: 817 groups raised for 55 d, and Mahuang and Tuer chickens raised for 60, 65, 70, and 75 days (d). After the completion of rearing period, the chickens were slaughtered, and 5 carcasses per group were randomly selected for SC manufacturing. Meat quality was determined based on product yield, pH, color, meat tenderness, and textural and sensorial attributes. The volatile compounds of chicken breast were identified by gas chromatography-ion mobility spectrometry (GC-IMS). The results showed that the yellow-feathered chicken breed, especially Mahuang chicken, had a higher product yield, and lower shear force and sensorial scores than the cross broiler. The pH, L* and b* values in SC breast meat were not significantly influenced by breed (P > 0.05), while greater a* was observed in SC of yellow-feathered chickens compared to cross broilers. The slaughter age had a significant effect on the pH, color, shear force, and textural properties of SC (P < 0.05). The meat tenderness of SC was significantly decreased as the age of chicken increased from 65 d to 75 d (P < 0.05). The relatively young age of yellow-feathered chickens (60 d and 65 d) was rated to have a higher overall sensory score of SC (P < 0.05). A total of 65 organic volatile compounds were identified in SC, including 18 aldehydes, 16 alcohols, 10 ketones, 9 esters, 2 acids, 3 furans, 5 pyrazines, and 2 sulfur-containing compounds. Three chicken breeds were separately clustered in the plot of principal component analysis, indicating breed-specific flavor characteristics. Collectively, the present study provides valuable information for SC processing in terms of carcass selection of yellow-feathered chicken breeds and slaughter age.
Key words: soft-boiled chicken, sensory evaluation, meat tenderness, gas chromatography-ion mobility spectrometry, principal component analysis
INTRODUCTION
Soft-boiled chicken (SC), also named balanced chicken or White-Cut chicken, is a typical cuisine and is commonly consumed during festivals in Asia due to its high reputation with hundreds of years of history (Xu et al., 2020). In pace with the increasing demand of the consumption market, SC has become an industrialized poultry meat product in recent years. Yellow-feathered broilers, with a proportion of approximately 32% of chicken production in China (2020), are usually used as the raw material for SC manufacturing. Due to the simple technological process with only boiling and cold-dipping, SC is favored and characterized by retaining original flavor, tender profiles, and white flesh with a slight oil yellow appearance of raw chicken (Deng et al., 2020). Similar to the consumption custom of pork meat, most of the Chinese choose SC processed with hot-fresh chicken carcass, although chilled chicken has been reported for benefits of SC flavor development (Wang et al., 2019; Xu et al., 2021).
The most important factors affecting the quality attributes of poultry meat and meat products are breed and slaughter age. Color, water holding capacity (WHC), tenderness, textural properties, and sensory evaluation have been widely considered for meat quality determination. Jayasena et al. (2013) investigated the differences in quality traits of Korean traditional cuisines, that is, samgyetang and baeksuk, between native chickens (Hanhyup) and broilers and found that color and pH were affected by chicken breed, while WHC and overall sensory quality were not different between chicken sources. Sarsenbek et al. (2013) showed that an indigenous breed of Baicheng-You chicken presented variable pH with 45 min of cooking loss of chicken breast with Arbor Acres broilers, but no significant difference in flesh color, drip loss, or shear force was observed between the two breeds. Moreover, Dyubele et al. (2010) indicated that consumers rated higher sensory scores including initial impression of juiciness, first bite, and sustained impression of juiciness for broiler chicken meat than for indigenous chicken meat from South Africa. In addition, the age also plays a critical role in meat quality. In a report of Korat hybrid chickens, the WHC and shear force of chicken breast increased and the pH of thigh meat decreased as the age of the chicken increased (Katemala et al., 2021). Wideman et al. (2016) suggested that the meat from older animals exhibited a darker color because the myoglobin content increased with age. Indigenous yellow-feathered chickens are the most preferred meat-type chickens in China, among which Mahuang and Tuer chickens are representative of the local chicken breed from Guangdong Province in South China. They are often regarded as raw materials for the preparation of SC at in kitchens and also in a preliminary attempt at industrial production (Deng et al., 2020). Previous investigations on yellow-feathered chickens have aimed to obtain fundamental data to improve growth performance and carcass traits by rearing systems (Wang et al., 2021) and dietary supplements (El-Senousey et al., 2019). Thus far, little scientific information is available regarding the edible quality of SC as influenced by the breed and age of chicken.
The flavor profile is one of the most important indicators of the sensory characteristics and influences consumer preference and acceptance of meat products (Jayasena et al., 2013). To date, more than 600 volatile compounds, including aldehydes, ketones, alcohols, acids and esters, and sulfur-containing compounds have been identified in cooked chicken products (Feng et al., 2018). Compared to the commonly used technique of gas chromatography-ion mobility spectrometry (GC-IMS) has been increasingly utilized to characterize volatile compounds. GC-IMS identifies chemical substances based on the migration rate of ionized molecules at ambient pressures and temperature under an electric field with characteristics of a lower detection limit, high sensitivity, and ease of use (Shvartsburg, 2010). Recently, the diversity of the flavor substances in Dezhou braised chicken at various stages of a meat processing line was profiled by using GS-IMS and 2-ethylhexanol was identified as a key flavor in the chicken carcasses (Yao et al., 2022). Xu et al. (2021) found 8 volatile aromas in Qingyuan partridge chicken as important ingredients forming the flavor of SC by GS-IMS fingerprint analysis. However, there are few reports on the effect of breed and age on the flavor volatile compounds of poultry meat and meat products, in this case, SC, in which typical volatile components have not been established.
Therefore, the objective of this research was to investigate the effect of Chinese indigenous yellow feather broiler breeds (Mahuang and Tuer chickens) and their age on meat quality and flavor profile of SC, compared with those of commercial 817 cross broiler. Our findings will facilitate the selection of chickens for SC manufacturing and will provide basic knowledge for a better understanding of the flavor characteristics of traditional poultry meat products.
MATERIALS AND METHODS
Ethics Statement
All the experimental procedures, including bird husbandry, management, and slaughtering were performed according to the protocol approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University (Protocol NO.: SYXK 2017-007).
Bird Husbandry and Slaughtering
One-day old commercial meat-type broiler (817 Crossbred broiler) and 2 Wens Yellow-feathered chickens (Mahuang and Tuer) were acquired from Southern Poultry Breeding Company of Wens Co. Ltd. (Yunfu, Guangdong, China). A total of 432 chicks were used and randomly allocated into 9 treatment groups: 1) 817 group (raised for 55 d), 2) MH55 group (Mahuang chicken raised for 55 d), 3) MH60 group (Mahuang chicken raised for 60 d), 4) MH65 group (Mahuang chicken raised for 65 d), 5) MH70 group (Mahuang chicken raised for 70 d), 6) TE55 group (Tuer chicken raised for 55 d), 7) TE60 group (Tuer chicken raised for 60 d), 8) TE65 group (Tuer chicken raised for 65 d), and 9) TE70 group (Tuer chicken raised for 70 d). Each treatment group had 6 replicate pens and each pen had 8 chicks. All rearing practices and management were uniformly performed. The ingredient and nutrient composition of the basal diet are shown in Table 1, which was formulated to meet the recommended nutrient requirements (NRC, 1994). The birds were maintained on ad libitum feeding and water with a 12 h light-dark cycle during the experimental period. The ambient temperature was set to 33 to 35°C from d 1 to 3 and then reduced to the final temperature of 25°C at a rate of 1°C per day. After the completion of the rearing period, the chickens were transported to a commercial slaughterhouse and were slaughtered according to the Operating Procedure for Slaughter of Livestock and Poultry-chicken in China (GB/T 19478-2018). A total of 45 chicken carcasses (5 carcasses per group) within 1 h postslaughter of each treatment group were randomly selected and used for SC processing.
Table 1.
Ingredients and nutrient composition of the basal diet.
| Ingredient | Starter1 | Grower2 | Finisher3 |
|---|---|---|---|
| Corn | 618.99 | 677.71 | 711.48 |
| Soybean meal (46%) | 229.89 | 140.69 | 100 |
| Cottonseed meal (46%) | 50 | 60 | 60 |
| Corn gluten meal (60%) | 50 | 57.12 | 61.2 |
| Soybean oil | 10.2 | 21 | 26.6 |
| Limestone | 13.7 | 14.3 | 14.3 |
| Dicalcium phosphate | 12.4 | 10.3 | 8.7 |
| Sodium chloride | 3.5 | 3.5 | 3.6 |
| Choline chloride (60%) | 0.8 | 0.5 | 0.3 |
| Premix4 | 4 | 4 | 4 |
| Lysine (70%) | 4.49 | 6.86 | 6.83 |
| Methionine (98%) | 1.29 | 2.38 | 1.46 |
| Threonine (98%) | 0.34 | 1.24 | 1.13 |
| Mold inhibitor | 0.4 | 0.4 | 0.4 |
| Calculated nutrient content | |||
| Metabolizable energy (kcal/kg) | 2,900 | 3,030 | 3,100 |
| Crude protein | 20.5 | 18 | 17 |
| Calcium | 0.9 | 0.85 | 0.8 |
| Phosphorus | 0.59 | 0.53 | 0.49 |
| Available phosphorus | 0.351 | 0.31 | 0.28 |
| Lysine | 1.05 | 0.97 | 0.88 |
| Methionine + cysteine | 0.71 | 0.76 | 0.65 |
| Threonine | 0.65 | 0.63 | 0.58 |
| Arginine | 1.22 | 1.02 | 0.92 |
The starter phase of 817 crossbred broilers is 1–21 d. The starter phase of Wens yellow-feathered Mahuang and Tuer chickensis 1–25 d.
The grower phase of 817 crossbred broilers is 22–42 d. The grower phase of Wens yellow-feathered Mahuang and Tuer chickens is 26–55 d.
The finisher phase of 817 crossbred broilers is 43–55 d. The finisher phase of Wens yellow-feathered Mahuang and Tuer chickens is 56–75 d.
The Premix provided per kilogram of the diet: Cu, 5.00 mg; Fe, 69.00 mg; Zn, 84.00 mg; Mn, 98.6 mg; I, 1.14 mg; Se, 0.30 mg; vitamin A (retinyl acetate), 15,000 IU; vitamin D (cholecalciferol), 3,000 IU; vitamin E (dl-α-tocopheryl acetate), 25.5 IU; vitamin K3, 2.1 mg; vitamin B1, 2.4 mg; vitamin B2, 9 mg; vitamin B6, 5.1 mg; vitamin B12, 0.02 mg; Calpan, 12 mg; niacin, 48 mg; folic acid, 1.2 mg; biotin, 0.06 mg; Roxarsone, 50 mg; salinomycin, 90 mg.
Preparation of Soft-Boiled Chicken
The SC was prepared according to the procedure described by Xu et al. (2021). Briefly, the whole chicken carcasses were immersed into a water bath of 90°C for 60 s and then, the residual blood on the skin was rinsed with chilled water (4°C). Next, the chickens were stewed in 2% brine at 85°C for 60 min and then stepwise placed into an icy water container for 20 min, and a 0 to 4°C cooler for air-cooling of 20 min to reduce the moisture content. The right-side chicken of breast and thigh were assigned for pH, color, tenderness, and texture profile analysis (TPA). The chicken breast part was usually preferred by consumer and the flavor of breast was better than other parts (Xu et al., 2020), the left-side chicken breast was used for sensory evaluation and GC-IMS analysis.
Yield
The whole chicken carcass before processing was weighed and the weight was recorded as the m1. The SC after the completion of air-chilling was weighed again and recorded as m2. The yield was calculated according to the following formula: Yield (%) = m2 / m1 × 100.
Determination of pH
The pH value of SC breast and thigh meat was determined by using a portable pH meter (Testo 205, Testo, Germany). Before use, the pH meter was calibrated with standard phosphate buffer (pH 4.00 and 7.00). Then, the probe of meter was inserted inside the chicken breast at 3 randomly selected locations, ensuring that the probe part was in full contact with meat samples. The values were recorded after the pH meter reading was stabilized. The pH value of each meat sample was obtained by the average of triplicate measurements.
Color Detection
The color was determined by using a colorimeter (CR-400, Konica Minolta, Japan) and calibrated with a white calibration plate. The colorimeter had an illuminant D65, a 10° standard observer position and an 8.0-mm diameter aperture. The instrumental color attributes including lightness (L*), redness (a*), and yellowness (b*) values of the meat samples were measured. The L*, a* and b* value was obtained by triplicate measurements randomly from different sites of each meat sample.
Warner-Bratzler Shear Force Detection
The Warner-Bratzler shear force (WBSF) of SC breast and thigh meat was determined by referring to the method of Zheng et al. (2007) with a slight modification. Three rectangular meat slices (1 cm × 1 cm × 4 cm) were cut along with the fiber direction of meat sample. The meat slices were sheared perpendicular to the muscle fiber orientation using a Texture Analyzer (C-LM3B, Northeast Agricultural University, Harbin, China). The testing speed was 200 mm/min. The maximum peak shear force value of each meat sample was recorded and the WBSF was expressed as kg.
Texture Profile Analysis
The TPA of processed chicken breast meat was assessed by the method of Zheng et al. (2015) with some modifications. Three meat samples were cut from SC breast and thigh meat and shaped into 1 cm × 1 cm × 1 cm cubes. The cube was placed on the center of worktable in the texture analyzer (TA-XT plus, Stable Micro Systems Co., Ltd., London, UK). The probe (SMP P/75, flat bottom, diameter 75 mm) was used to compress the samples to 50% of its original height by using a double compression cycle at room temperature. The parameters were set as follows: the trigger force of 5 g, a pre-test speed of 2 mm/s, a test speed of 1 mm/s and a post-test speed of 3 mm/s. The hardness, springiness, cohesiveness, chewiness, and resilience were obtained from the exponent software (Exponent Stable Microsystem, version 5.1.2.0, Stable Micro Systems Co., Ltd.). TPA attributes of each chicken breast were averaged from triplicate measurements.
Sensory Evaluation
The sensory evaluation was conducted by a trained sensory panel employed from our previous study (Deng et al., 2020). Briefly, there were 10 panelists (5 males and 5 females), aged between 22 and 45 years old with experience of chicken production and processing for years in R&D department of Guangdong Wen's Caren Foodstuffs Co., Ltd (Yunfu, Guangdong, China). The panelist training, sensory room, and procedures of sensory evaluation followed the Chinese standard GB/T 22210-2008 (Criterion for sensory evaluation of meat and meat products). Several key points needed to be outlined: 1) Trained sensory panelists were able to recognize and score the appearance, texture, odor, taste, and overall acceptability based on 9-point scale as shown in Table 2. 2) Panelist was set in individual compartment and all forms of communication were not allowed during evaluation. 3) Smoking, drinking, and eating were not allowed at least 1 h before sensory analysis. 4) The meat sample (3 g) of each chicken breast at room temperature was placed on a white ceramic plate labeled with three-digit random code. 5) The distilled water was provided for panelists to rinse their months to avoid the cross-links effects. All the data were collected and used for statistical analysis.
Table 2.
The definition of sensory quality attributes.
| Attributes | Definition |
|---|---|
| Appearance | Visual judgement of meat surface whether the flesh with a slight oily-yellow is uniform and shiny (uniform and shiny meat surface = 9, bloody spots = 1) |
| Texture | Oral processing of meat whether it is tight and chewy with typical characteristic of springiness and tender (easy to chew, springiness, and tender = 9, tough, dried, hard to chew = 1) |
| Odor | Smell the meat whether it has meaty flavor or off-odor (Strong meaty odor = 9, off-odor = 1) |
| Taste | Tongue response to meat during chewing whether it has chicken flavor and taste, unique umami of soft-boiled chicken (Strong chicken flavor and taste = 9, bloody taste without unique umami taste and flavor = 1) |
| Overall acceptability | General feeling of meat whether it is acceptable (like extremely = 9, dislike extremely = 1) |
GC-IMS Analysis
The volatile compounds of SC breast meat were detected by using a GC-IMS flavor analyzer (FlavourSpec, G.A.S., Dortmund, Germany), which was described by with a slight modification. The meat sample was mined and 2 g was weighted to place into a 20 mL headspace vial. Then, the vial was incubated at 75°C for 15 min and 500 μL of sample was injected via a heated syringe at 85°C in the automatic headspace injection system (TriPlus RSH, Thermo Fisher Scientific Co., Ltd., Waltham, MA). The analytes were separated with a MXT-WAX column (0.53 mm × 30 m, 1 μm, Restek Corporation, Bellefonte, PA) at 60°C. Nitrogen (purity 99.9%) was used as the carrier and drift gas. The carrier gas flow rate was 2 mL/min for initial 2 min, and then increased to 10 mL/min in next 8 min, and finally increased to 100 mL/min within 10 min and held for 10 min. The flow rate of gas in the drift tube was 150 mL/min. The flavor compounds were identified by using the drift time (RIP) and retention index (RI), which was normalized with the external references of n-ketones C4–C9, and matched with the standards in the GC-IMS library. The laboratory analysis viewer (LAV) software (G.A.S.) was used to collect and analyze the data and gallery plots of the volatile compounds in each group were constructed.
Statistical Analysis
The statistical analyses were performed using the Statistical Analysis System (SAS 9.0, SAS Institute Inc., NC). Data were evaluated using a mixed-model ANOVA with the breed and age as fixed effects and breed × age as interaction effect, regarding the chicken as a random factor. The significance of differences among the group means was assessed using Duncan's multiple range test. All the results were presented as means ± standard error (SE), and significance level was set at P < 0.05. Principal component analysis (PCA) was performed on meat quality attributes and volatile compounds using SIMCA-P software (V.14.1).
RESULTS
Yield
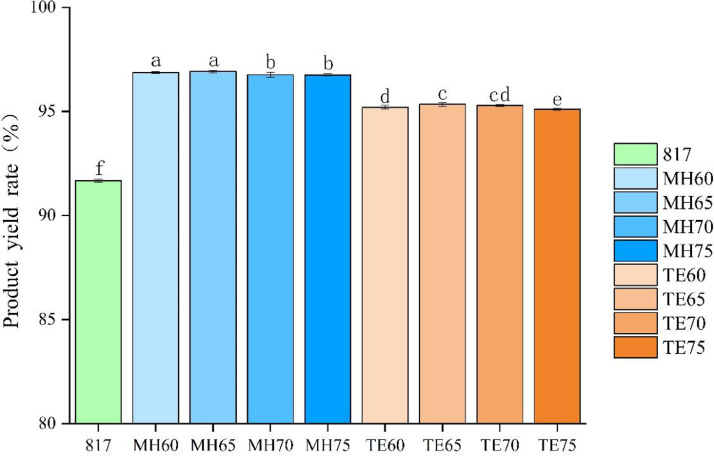
As shown in Figure 1, breed showed a significant effect on the SC product, among which Mahuang chickens exhibited the highest yield, followed by Tuer chickens and 817 cross broilers presented the lowest yield (P < 0.05). Compared to breed, the age of the chicken showed minimal effect on product yield. The Mahuang chickens at the ages of 60 and 65 d presented slightly higher yields than those at of 70 and 75 d (P < 0.05), and Tuer chickens at the ages of 65 and 75 d showed higher yields than those at 60 and 75 d (P < 0.05).
Figure 1.
The effect of breed and age on product yield of soft-boiled chickens. The 817 indicated 817 cross broiler raised for 55 d. MH60, MH65, MH70, and MH75 represented Mahuang chicken that were raised for 60, 65, 70, and 75 d, respectively. TE60, TE65, TE70, and TE75 represented Tuer chicken that was raised for 60, 65, 70, and 75 d, respectively. Different letters indicated significant difference among treatment groups at P < 0.05.
pH and Color
The pH and color of SC breast and thigh meat are presented in Tables 3 and 4, respectively. The breed showed no significant effect on pH value (P > 0.05), while pH of SC breast and thigh meat was significantly affected by age (P < 0.05). The pH in the MH70 and MH75 groups was lower than that of the MH60 and MH65 groups by approximately 0.05 pH units in breast meat and 0.10 pH units in thigh meat, respectively. For the Tuer breed, the TE60 group had the lowest pH of chicken breast among all treatments with 0.11 pH units lower than the TE65 group. Moreover, the pH values of thigh meat in Tuer chickens at the ages of 65 and 70 d were significantly higher than those at 60 and 75 d (P < 0.05). The interaction effect between breed and age was also observed to have a significant influence on the pH value of SC meat (P < 0.05). As shown in Table 4, both L* and b* values in chicken breast meat and b* in chicken thigh meat were not significantly influenced by breed (P > 0.05), while age significantly affected L*, a*, and b* in SC breast and thigh meat (P < 0.05). Generally, the Mahuang chicken at the age of 65 d possessed lower L* and higher a* and b* values than other feeding periods in the Mahuang group. No significant difference was found in the color attributes of Mahuang chicken breast and thigh meat at the ages of 70 and 75 d (P > 0.05). A relatively higher a* value in the TE65 group was found in chicken breast and thigh meat whereas a* in the TE60 group was the lowest in the Tuer breed. Minimal differences in quantitative values were found in L* and b* in the Tuer breed at various ages.
Table 3.
The effect of breed and age on pH of soft-boiled chicken breast and thigh meat.
| Breed (B) | Age (D) | Breast | Thigh |
|---|---|---|---|
| 817 | 55 | 6.24 ± 0.04a | 6.76 ± 0.07bc |
| Mahuang | 60 | 6.24 ± 0.03a | 6.79 ± 0.05ab |
| 65 | 6.25 ± 0.04a | 6.80 ± 0.04ab | |
| 70 | 6.19 ± 0.02b | 6.72 ± 0.03c | |
| 75 | 6.19 ± 0.02b | 6.71 ± 0.02cd | |
| Tuer | 60 | 6.13 ± 0.05c | 6.72 ± 0.08cd |
| 65 | 6.24 ± 0.02a | 6.82 ± 0.02a | |
| 70 | 6.21 ± 0.02ab | 6.80 ± 0.04ab | |
| 75 | 6.20 ± 0.02b | 6.70 ± 0.04d | |
| P-value | B | 0.0668 | 0.8095 |
| D | 0.0004 | 0.0002 | |
| B*D | 0.0001 | 0.0182 |
Different letters within each column indicated significant difference among treatment groups at P < 0.05 (n = 5).
Table 4.
The effect of breed and age on color attributes of soft-boiled chicken breast and thigh meat.
| Breed (B) | Age (D) | Breast |
Thigh |
||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | ||
| 817 | 55 | 78.13 ± 0.87a | 2.09 ± 0.05d | 11.42 ± 0.86c | 63.41 ± 1.07b | 5.45 ± 0.16e | 12.95 ± 0.47a |
| Muhuang | 60 | 76.86 ± 0.51bcd | 2.48 ± 0.13cd | 12.41 ± 0.26ab | 62.18 ± 1.57bc | 6.53 ± 0.27b | 11.42 ± 0.28cd |
| 65 | 73.94 ± 0.65e | 3.43 ± 0.30a | 12.75 ± 0.62a | 58.56 ± 2.14c | 7.50 ± 0.51a | 12.83 ± 1.18a | |
| 70 | 76.72 ± 0.36bcd | 3.15 ± 0.37a | 10.29 ± 0.42d | 66.28 ± 1.79a | 5.73 ± 0.30cd | 11.43 ± 1.06cd | |
| 75 | 77.79 ± 1.01ab | 3.11 ± 0.23ab | 10.30 ± 0.94d | 65.77 ± 1.84ab | 5.81 ± 0.33cd | 12.07 ± 0.73abc | |
| Tuer | 60 | 76.25 ± 1.32d | 2.39 ± 0.38cd | 12.58 ± 0.79a | 58.85 ± 1.69c | 5.60 ± 0.17de | 12.20 ± 0.10abc |
| 65 | 75.03 ± 0.73e | 3.00 ± 0.17abc | 11.66 ± 0.48bc | 56.84 ± 1.59d | 6.04 ± 0.28c | 12.39 ± 1.10ab | |
| 70 | 76.52 ± 1.12cd | 2.84 ± 0.13abc | 11.02 ± 0.48cd | 59.18 ± 1.09c | 5.64 ± 0.19de | 10.82 ± 0.60d | |
| 75 | 77.39 ± 0.87abc | 2.61 ± 0.18bcd | 11.61 ± 0.67bc | 61.24 ± 1.70bc | 5.74 ± 0.24cd | 11.50 ± 0.76bc | |
| P-value | B | 0.9174 | 0.0001 | 0.1865 | <0.0001 | <0.0001 | 0.4356 |
| D | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0049 | |
| B*D | 0.1444 | 0.2681 | 0.0016 | 0.1061 | <0.0001 | 0.2284 | |
Different letters within each column indicated significant difference among treatment groups at P < 0.05 (n = 5).
WBSF
The meat tenderness as indicated by WBSF was determined on SC breast and thigh meat. As presented in Table 5, the effects of breed, age, and breed × age were significant for WBSF of chicken meat (P < 0.05). Compared with the 817 cross broiler, Mahuang and Tuer chickens had lower shear force of breast and thigh meat, among which Mahuang chickens at the age of 65 d were shown to have the lowest WBSF value among the 9 treatment groups. There was no significant difference in meat tenderness between the MH60 and MH65 groups (P > 0.05). The WBSF of Mahuang and Tuer chicken breast and thigh meat increased with the extension of the feeding period from 65 to 75 d. In addition, thigh meat was found to have a lower WBSF value than breast meat.
Table 5.
The effect of breed and age on Warner-Bratzler shear force of soft-boiled chicken breast and thigh meat.
| Breed (B) | Age (D) | Breast | Thigh |
|---|---|---|---|
| 817 | 55 | 5.25 ± 0.08a | 4.56 ± 0.15a |
| Mahuang | 60 | 3.45 ± 0.06de | 2.36 ± 0.08e |
| 65 | 3.25 ± 0.07e | 2.27 ± 0.11e | |
| 70 | 3.70 ± 0.16cd | 2.79 ± 0.15d | |
| 75 | 3.91 ± 0.13c | 3.05 ± 0.10cd | |
| Tuer | 60 | 3.56 ± 0.10d | 2.86 ± 0.07cd |
| 65 | 3.41 ± 0.12e | 2.47 ± 0.07e | |
| 70 | 4.34 ± 0.08b | 3.06 ± 0.19c | |
| 75 | 4.51 ± 0.07b | 3.56 ± 0.07b | |
| P-value | B | <0.0001 | <0.0001 |
| D | <0.0001 | <0.0001 | |
| B*D | <0.0001 | 0.0074 |
Different letters within each column indicated significant difference among treatment groups at P < 0.05 (n = 5).
Textural Properties
The textural attributes of SC breast and thigh meat were determined and are shown in Tables 6 and 7, respectively. The breed and age showed a significant effect on the hardness of chicken breast and thigh meat (P < 0.05). The hardness of chicken breast meat in all ages of Mahuang breed and Tuer breed below 70 d of age was significantly lower than that of 817 cross broiler (P < 0.05), which showed no significant difference with Tuer chicken at 75 d (P > 0.05). For thigh meat, the Mahuang and Tuer breeds showed the lowest hardness at the age of 65 d, however, Tuer chickens at the age of 75 d possessed the highest hardness. Consistent with meat shear force, greater hardness in breast meat was detected in comparison to thigh meat and both of them increased during the extension of the feeding period. With respect to springiness, no variable difference was observed for the 3 breeds (P > 0.05). Mahuang and Tuer chickens presented the lowest springiness at the age of 60 d and the highest springiness at the age of 65 d. Both the cohesiveness and resilience of breast meat were not affected by the breed and age (P >0.05); however, the lowest values in thigh meat at the age of 65 d were observed for both the Mahuang and Tuer chicken breeds. Likewise, the chewiness of chicken meat showed the lowest value at the age of 65 d and increased with the feeding period.
Table 6.
The effect of breed and age on texture properties of soft-boiled chicken breast meat.
| Breed (B) | Age (D) | Hardness/g | Springiness/mm | Cohesiveness/ratio | Chewiness/mJ | Resilience/ratio |
|---|---|---|---|---|---|---|
| 817 | 55 | 3,071.73 ± 74.52a | 0.63 ± 0.04ab | 0.43 ± 0.04c | 781.35 ± 32.78b | 0.15 ± 0.02b |
| Mahuang | 60 | 2,601.45 ± 42.70d | 0.55 ± 0.04d | 0.46 ± 0.05b | 705.97 ± 8.51c | 0.16 ± 0.03ab |
| 65 | 2,339.87 ± 95.96f | 0.63 ± 0.04a | 0.46 ± 0.05ab | 671.38 ± 13.30c | 0.16 ± 0.02ab | |
| 70 | 2,626.41 ± 54.09cd | 0.59 ± 0.03abc | 0.45 ± 0.03b | 806.11 ± 34.16ab | 0.16 ± 0.02ab | |
| 75 | 2,750.52 ± 43.68bc | 0.52 ± 0.01d | 0.46 ± 0.03ab | 832.56 ± 38.96a | 0.17 ± 0.01a | |
| Tuer | 60 | 2,579.80 ± 40.59de | 0.59 ± 0.03bc | 0.46 ± 0.03ab | 707.23 ± 7.01c | 0.16 ± 0.01ab |
| 65 | 2,467.41 ± 58.90ef | 0.60 ± 0.04abc | 0.43 ± 0.04bc | 673.38 ± 28.21c | 0.15 ± 0.01b | |
| 70 | 2,760.37 ± 43.75b | 0.57 ± 0.02c | 0.46 ± 0.04ab | 793.26 ± 13.94b | 0.16 ± 0.02ab | |
| 75 | 3,133.40 ± 68.62a | 0.57 ± 0.01c | 0.49 ± 0.02a | 829.35 ± 41.79a | 0.16 ± 0.01ab | |
| P-value | B | <0.0001 | 0.5251 | 0.8482 | 0.7223 | 0.5825 |
| D | <0.0001 | 0.0009 | 0.5416 | <0.0001 | 0.3359 | |
| B*D | <0.0001 | 0.0215 | 0.4449 | 0.9303 | 0.6364 |
Different letters within each column indicated significant difference among treatment groups at P < 0.05 (n = 5).
Table 7.
The effect of breed and age on texture properties of soft-boiled chicken thigh meat.
| Breed (B) | Age (D) | Hardness/g | Springiness/mm | Cohesiveness/ratio | Chewiness/mJ | Resilience/ratio |
|---|---|---|---|---|---|---|
| 817 | 55 | 1,534.37 ± 37.36c | 0.63 ± 0.05ab | 0.46 ± 0.04ab | 477.94 ± 17.83c | 0.19 ± 0.03a |
| Mahuang | 60 | 1,322.40 ± 68.54d | 0.56 ± 0.07bc | 0.46 ± 0.03ab | 441.74 ± 21.09d | 0.17 ± 0.02ab |
| 65 | 1,163.08 ± 32.51e | 0.65 ± 0.07a | 0.28 ± 0.02e | 384.94 ± 13.40e | 0.15 ± 0.03bc | |
| 70 | 1,618.70 ± 49.63bc | 0.59 ± 0.05abc | 0.49 ± 0.03a | 541.88 ± 41.29b | 0.21 ± 0.03a | |
| 75 | 1,570.28 ± 15.34c | 0.59 ± 0.09abc | 0.44 ± 0.07abc | 617.09 ± 29.64a | 0.18 ± 0.04ab | |
| Tuer | 60 | 1,410.10 ± 52.54d | 0.51 ± 0.04c | 0.40 ± 0.06bcd | 540.40 ± 6.78b | 0.16 ± 0.02b |
| 65 | 1,240.02 ± 42.45de | 0.63 ± 0.05ab | 0.34 ± 0.07de | 465.47 ± 26.56cd | 0.14 ± 0.02c | |
| 70 | 1,694.05 ± 69.24b | 0.58 ± 0.06abc | 0.37 ± 0.05cd | 470.37 ± 34.08cd | 0.15 ± 0.02bc | |
| 75 | 2,115.13 ± 67.90a | 0.61 ± 0.09ab | 0.45 ± 0.12ab | 534.67 ± 24.26b | 0.20 ± 0.02a | |
| P-value | B | <0.0001 | 0.4908 | 0.1740 | 0.4569 | 0.1249 |
| D | <0.0001 | 0.0096 | <0.0001 | <0.0001 | 0.0297 | |
| B*D | <0.0001 | 0.7033 | 0.0164 | <0.0001 | 0.0453 |
Different letters within each column indicated significant difference among treatment groups at P < 0.05 (n = 5).
Sensory Evaluation
The sensory attributes including appearance, texture, odor, taste, and overall acceptability are shown in Table 8. Compared to the 817 cross broiler chickens, the Mahuang and Tuer chickens had higher scores of appearance, while there were no variable differences between the 2 breeds at the 4 different ages. Both breed and age showed significant effects on texture, odor, taste, and overall acceptability (P < 0.05). Generally, Mahuang chickens presented the highest scores, followed by the Tuer chickens, and 817 cross broilers had the lowest scores. During the feeding period, the score at the age of 65 d was the highest, followed by the scores at the ages of 60 d and 70 d, while the score at the age of 75 d was the lowest. Overall, it can be suggested that the Mahuang chickens at the ages of 60, and 65 d and Tuer chickens at the age of 65 d were acceptable for typical characteristics of SC by sensory evaluation.
Table 8.
Sensory rating scores for soft-boiled chicken of different treatment groups.
| Breed (B) | Age (D) | Appearance | Texture | Odor | Taste | Overall acceptability |
|---|---|---|---|---|---|---|
| 817 | 55 | 5.86 ± 0.49d | 6.24 ± 0.53c | 6.40 ± 0.72bc | 4.66 ± 0.29e | 6.40 ± 0.34c |
| Mahuang | 60 | 8.58 ± 0.50a | 8.14 ± 0.59ab | 7.74 ± 0.41ab | 7.32 ± 0.21b | 8.00 ± 0.31a |
| 65 | 8.26 ± 0.34abc | 8.38 ± 0.33a | 8.00 ± 0.47a | 8.42 ± 0.17a | 8.48 ± 0.20a | |
| 70 | 7.98 ± 0.38abc | 7.02 ± 0.29bc | 7.88 ± 0.39ab | 7.06 ± 0.34bc | 7.86 ± 0.26a | |
| 75 | 7.68 ± 0.43abc | 5.64 ± 0.45c | 6.52 ± 0.39abc | 6.14 ± 0.43cd | 6.40 ± 0.26c | |
| Tuer | 60 | 7.82 ± 0.51abc | 6.12 ± 0.32c | 6.38 ± 0.57bc | 5.46 ± 0.25de | 6.94 ± 0.34bc |
| 65 | 8.42 ± 0.28abc | 6.88 ± 0.40bc | 7.06 ± 0.50abc | 6.40 ± 0.26cd | 7.60 ± 0.34ab | |
| 70 | 7.06 ± 0.30c | 6.86 ± 0.53bc | 6.94 ± 0.34abc | 6.26 ± 0.34cd | 6.82 ± 0.12bc | |
| 75 | 7.22 ± 0.39b | 6.12 ± 0.37c | 5.98 ± 0.37c | 5.88 ± 0.32d | 6.66 ± 0.29c | |
| P-value | B | 0.1032 | 0.0147 | 0.0087 | <0.0001 | 0.0015 |
| D | 0.0761 | 0.0023 | 0.0506 | 0.0005 | <0.0001 | |
| B*D | 0.5978 | 0.0213 | 0.8708 | 0.0177 | 0.0687 |
Different letters within each column indicated significant difference among treatment groups at P < 0.05.
Volatile Flavor Compounds
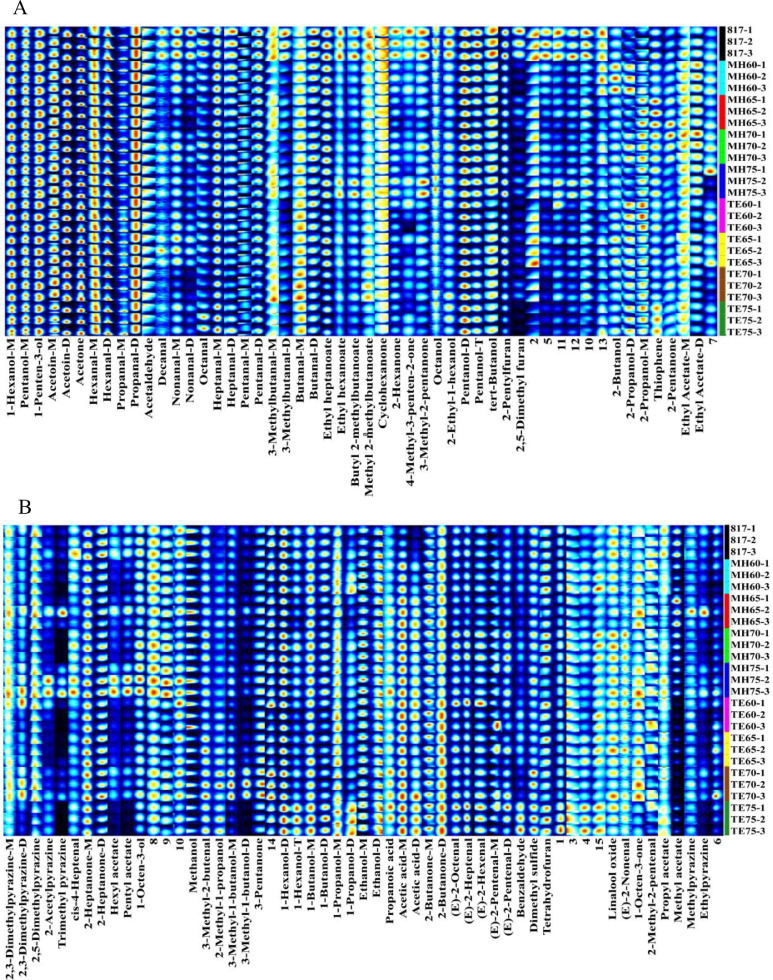
The gallery plot was constructed to intuitively compare the differences in volatile flavor compounds among the 9 treatment groups. As shown in Figure 2, the point in the fingerprint spectrum represents a volatile compound, which is annotated below, and the brightness of the color indicated the intensity of the substance. A total of 65 organic volatile compounds were identified, including 18 aldehydes, 16 alcohols, 10 ketones, 9 esters, 2 acids, 3 furans, 5 pyrazines, and 2 sulfur-containing compounds. In addition, another 15 compounds shown as numbering in Figure 2 were also observed but unmatched with the identified volatile library. The comparison of identified flavor compounds among treatment groups is presented in supplementary material Table S1. Several volatile compounds had different forms, such as protonated monomers (M) and proton-bound dimers (D), which exhibited with similar molecular formulas and odor descriptions, thus they were grouped together for relative intensity quantification.
Figure 2.
Fingerprint of characteristic volatile flavor compounds (A and B) in soft-boiled chickens corresponding different treatment groups.
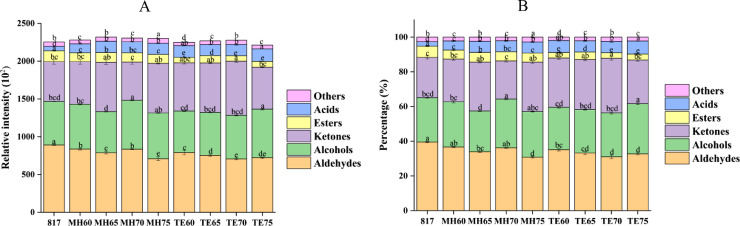
The relative peak area intensity and percentage of volatile compound categories were compared among the treatment groups (Figure 3). The aldehyde intensity and percentage in the 807 cross broiler group were higher than those of the Mahuang and Tuer breeds, which was indicated by the strong signal intensities of heptenal, hexanal, pentanal, heptanal and butanal. Some of the aldehydes including propanal, acetaldehyde, (E)-2-octenal, 3-methyl-2-butenal, and 2-methyl-2-pentenal were shown to have comparable relative intensities among the treatments groups. Notably, the Muhang and Tuer breeds showed a relatively higher content of (E)-2-pentenal than that of the 817 cross broiler, especially in the Tuer breed at the age of 60 d, which was 4.8 times that of the 817 group. Moreover, Mahuang aged at 75 d and Tuer chicken at 70 d showed the lowest content of aldehydes during their feeding period. Chicken age, rather than breed, showed a profound effect on the relative intensity and percentage of alcohols. The Mahuang chicken at 70 d and Tuer chicken at 75 d had the highest content of alcohols within their ages while Mahuang chicken aged at 65 d and Tuer chicken aged at 60 d showed the lowest. The top three alcohols were 1-hexanol, pentanol, and ethanol, which dominated the trend of alcohol differences among treatments. Compared to 817 broilers, 2 yellow-feathered chicken breeds showed higher contents of 1-propanol and 2-propanol, among which Mahuang chickens aged at 70 d exhibited 2.9 times the 1-propanol content than those aged at 817. Similarly, the highest 1-octen-3-ol was detected in Mahuang chickens aged at 75 d, which was 1.3 fold higher than that in the 817 breed. Not much variability was observed for 1-butanol, 2-butanol, octanol, and linalool oxide among the treatment groups.
Figure 3.
The relative intensity (A) and percentage (B) of volatile categories in soft-boiled chicken of corresponding treatment groups. The 817 indicated 817 cross broiler raised for 55 d. MH60, MH65, MH70, and MH75 represented Mahuang chicken that were raised for 60, 65, 70, and 75 d, respectively. TE60, TE65, TE70, and TE75 represented Tuer chickens that were raised for 60, 65, 70, and 75 d, respectively. Different letters indicated significant difference among treatment groups at P < 0.05.
With respect to ketones, the yellow-feathered chicken breed presented a higher content and percentage than the 817 broiler, except for Mahuang chickens aged at 70 d. In contrast, the highest ketone content and percentage were detected in Tuer chickens aged at 70 d, which was supported by the contents of acetoin, acetone, 2-butanone, pentanone, and 2-heptanone. Among them, acetoin in the MH65 group had the highest content of ketones in all groups, suggesting that it is a typical volatile of Mahuang chickens aged at 65 d. The highest content and percentage of esters were found in 817 cross broilers, followed by Mahuang chickens, and Tuer chickens possessed the lowest. This trend was in accordance with individual ester volatiles, including methyl acetate, hexyl acetate, ethyl hexanoate, and ethyl heptanoate. The ethyl acetate content was higher in the Mahuang group, followed by the 817 group and the Tuer group possessed the lowest ethyl acetate content. No obvious difference in esters was detected within the ages of the respective yellow-feathered breeds. Two acids, that is, acetic acid and propanoic acid were detected in SC. Compared to the 817 group, Mahuang and Tuer chickens showed a significantly higher relative intensity of acetic acid, while Tuer chickens aged at 75 d possessed a 3 times higher relative intensity than the 817 group. The furans, pyrazines and sulfur-containing compounds were detected to have low relative intensity in chicken meat; thus, they were combined as “others” in the Figure 2. The MH75 group showed the highest content, while the TE60 group was the lowest. The TE75 group exhibited the highest content of tetrahydrofuran, 2,5-dimethylfuran, 2 sulfur-containing compounds (dimethyl sulfide and thiophene), 2-pentylfuran and methylpyrazine in the 817 group, and MH75 possessed with the highest content of trimethyl pyrazine and 2-acetylpyrazine.
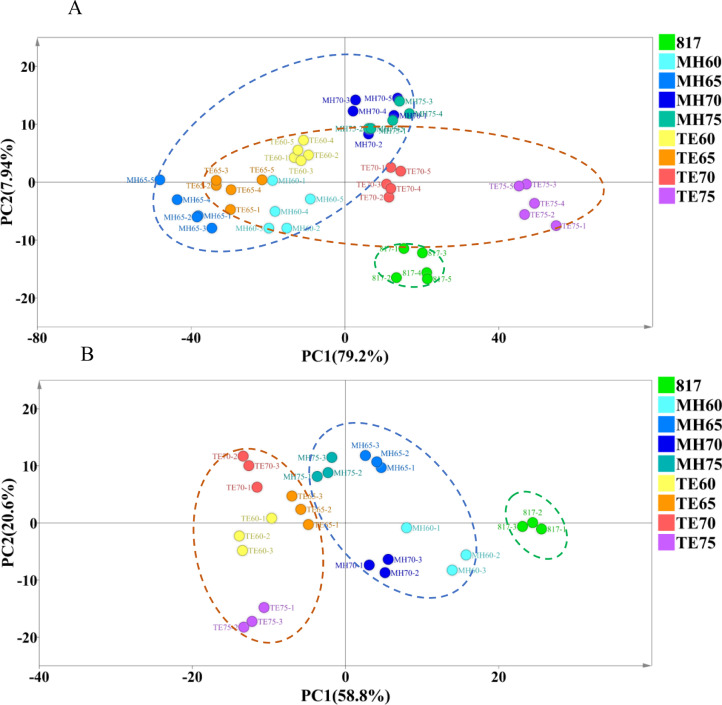
Principal Component Analysis
PCA was performed to further characterize the difference in meat quality and flavor among the treatment groups as shown in Figures 4A and 4B, respectively. All the measurements of meat quality attributes including yield, pH, color, WBSF, textual properties, and sensory scores were included in the PCA model, which extracted the first 2 PC1 and PC2, accounting for 87.14% of the total variance in the initial dataset (Figure 4A). The 817 group was scattered in quadrant IV near the negative PC2 axis, which was distinctive from the yellow-feathered chicken breed. The Mahuang and Tuer groups were shown to have partly overlapped in the PCA plot because MH60, MH65, TE60, and TE65 were closely clustered on the negative PC1 axis. The TE70 and TE75 groups were scattered along the positive PC1 axis and visibly separated. In addition, the MH70 and MH75 groups were located in the same region, indicating a similar meat quality of Mahuang aged at 70 and 75 d. Similarly, 65 volatile compounds were included in the PCA model of flavor analysis and the first 2 components accounted for 79.4% of the total variance. It was observed that the 3 breeds of chicken were separately clustered regardless of age, indicating breed-specific of flavor characteristics. Moreover, the age of Mahuang and Tuer chickens exhibited a significant effect on volatile compounds, showing with distinguishable scatter points in the PCA plot (Figure 4B).
Figure 4.
Scores scatter plot of soft-boiled chickens meat quality (A) and flavor compounds (B) by principle component analysis. The dotted blue circle indicated the Mahuang chicken group with different ages. The dotted orange circle indicated Tuer chicken groups with different ages. The dotted green circle indicated 817 group.
DISCUSSION
Selective breeding and feeding of chickens for high weight and carcass yield has been the standard throughout the poultry industry for decades to improve profits (Tallentire et al., 2016). Indeed, this strategy has promoted the development of fast-growing broilers, including Ross, Arbor Acres, and Cobb Broilers, for commercialization. Currently, consumers demand higher quality chickens with the rapid development of the economy and the increasing living standards. As such, native chickens, which are commonly slow-growing indigenous chickens, have received much attention. Local chicken strains are popular in Thai due to the disparate meat quality being chewy and tasty and a high human-health value; thus, consumers are willing to pay more, even 3 to 5 times more for native breeds chicken than for broiler meat (Jaturasitha et al., 2016). Similarly, Chinese people preferred yellow-feathered chickens. They are primarily sold in live poultry meat markets and wholesale wet market, while wet markets were restricted to cites in China due to the outbreak of animal influenza in 2015 (Deng et al., 2020), thus indirectly promoting the share of value-added poultry meat products. In this context, the chicken rearing has been proposed to be well adapted to meat processing. The current study was intended to investigate the effect of breed and age of yellow-feathered chicken on edible meat quality and flavor of SC to provide a guideline for SC meat product development to meet the demand of consumers and market requirements.
Product yield is an important parameter for economic value and also reflects the cooking loss of SC in the current study. As the SC was prepared with the whole chicken carcass, moisture, and meat exudates were largely retained during cooking resulting in the product yield of SC over 90%, which was much higher than that of the cooking yield of meat (appropriately 80%) due to the removal of surface connective tissue, skin, and fat of meat before cooking (PołTowicz and Doktor, 2012). It was noticed that the yellow feather broilers of all ages presented significantly higher product yields, indicating a better WHC than that of the 817 cross broiler. This finding agrees with those of previous studies, in which indigenous chicken breeds presented lower cooking loss than broiler chickens (Jaturasitha et al., 2008; Tang et al., 2009). Instrumental measurements of L*, a*, and b* values are indicators of color attributes. The amount and chemical state of myoglobin are determinant factors affecting the appearance of meat color (Mancini and Hunt, 2005). The thigh meat of SC had a higher a* and lower L* compared with that of breast meat of SC. This observation could be attributed to the higher myoglobin content, and higher proportion of type I and intermediate fibers in thigh meat compared to that of breast meat, which had higher white type II fibers (Wideman et al., 2016). Previous studies have shown that chicken meat color can be affected by strain and age (Abdullah et al., 2010). We found that SC prepared by yellow-feathered chickens exhibited relatively higher a* of breast and thigh meat than that of 817 cross broilers while no significant difference was observed for L* and b*. Jayasena et al. (2013) observed that the L* of chicken and thigh meat in samgyetang (a traditional Korean cuisine), but not a*, was significantly different between Korean native chickens and broilers, while in baeksuk (another cuisine), both L* and b* were affected by 2 strains. There have been many reports concerning the effect of breed on the color of fresh meat of chicken carcass, while both significant and insignificant effects has been indicated, possibly due to the genetic differences (Mehaffey et al., 2006; Brewer et al., 2012; Sarsenbek et al., 2013). Generally, the color of breast and thigh chicken meat tends to become darker and redder tonalities as bird ages (Fletcher, 2002), which is in agreement with our finding that a higher L* and lower a* at a young age of 60 d in yellow-feathered chickens are significantly changed with the extension of feeding to 65 to 75 d. The pH values are a vital value to affect both organoleptic and technological parameters of meat quality (Duclos et al., 2007). The pH of chicken breast and thigh meat in SC were at range of 6.13 to 6.24, 6.70 to 6.80, respectively, which were higher than those commonly observed in raw meat. The rise of meat pH during cooking was mainly associated with protein denaturation and charge changes due to the loss of acidic groups and accumulation of alkaline groups (Yarmand and Homayouni, 2010). In contrast to Tuer chickens, Mahuang chickens showed a tendency toward a decrease in the pH of chicken breasts with increasing age, which coincided with other reports (PołTowicz and Doktor, 2012).
Texture is a crucial attribute affecting consumer acceptance of meat products, with tenderness being the most important attribute (Shi et al., 2021). In general, slow-growing indigenous breeds have a substantially higher slaughter age of birds with more collagen crosslinkage, resulting in the likelihood of less tender meat compared to fast-growing broilers (Jaturasitha et al., 2016). Tang et al. (2009) compared the meat quality of 5 Chinese chicken breeds and revealed that birds from 3 slowing-growing genotypes (Wenchang, Xianju, Hy-line brown chicken, all at 16 wk of age) were detected to have higher shear force than those from fast-growing genotypes (Avian and Lingnanhuang, at 7 and 8 wk, respectively). Similarly, Thai indigenous chickens, Gallus domesticus at the age of 16 wk, had greater shear force in either raw or cooked meat than that of 38-day-old faster-growing Rhode Island Red broilers (Wattanachant et al., 2004). The current study also indicated that meat tenderness decreased with aging from 60 to 75 d in both breast and thigh meat of SC, as evidenced by the shear force, hardness, and chewiness measurements. However, the 817 cross broiler at slaughter age 55 d presented tough meat compared to yellow-feathered chicken with a slightly older age (60–65 d). This might be due to the difference in cooking yield such that 817 cross broiler chickens lost more meat moisture than Mahuang and Tuer chickens. On the other hand, the slaughter age with respect to the yellow-feathered chicken breed was still at a young age of 60 d, as Mahuang chickens were raised for 120 d on some local chicken farms in China (Wu et al., 2021). In addition, the Tuer chicken breed at 75 d showed comparable hardness of chicken breast meat with 817 cross broilers, and the chewiness in both Mahuang and Tuer chicken breast and thigh meat at 75 d was significantly higher than that of 817 cross broilers. It was conceivable that yellow-feathered chickens would become tougher as rearing age exceeds 75 d. Combined with sensory elevation, a relatively early age of marketing chickens was helpful in improving the meat texture of native chickens, which was also suggested by Sarsenbek et al. (2013).
Flavor is one of the major factors for consumer satisfaction with meat products and defined as a sensory impression sensed by taste and smell buds (Sitz et al., 2005). Flavor volatile compounds in cooked meat are formed are and mainly derived from the Maillard reaction, lipid oxidation, the interaction of those 2 processes and vitamin degradation. It is highlighted that the precursors including fatty acid composition, free amino acid, and nucleotide contents, especially inosine-5′-monophosphate (IMP), mostly contribute to flavor volatiles. The volatile precursors in chicken meat and meat products were affected by breed and age, which has been investigated in a vast number of studies (Jayasena et al., 2013). Previously, correlations were established between 8 volatile aromas and four free amino acids, 3 nucleotides, and seven low-molecular weight of water-soluble peptides, which were identified as the main compounds affecting the flavor of SC (Xu et al., 2021). Furthermore, the volatile compounds in five parts of SC (skin, breast, thigh, head, and butt) were characterized, in which chicken breast was shown to have a more chicken-like flavor than other parts and was preferred by sensory assessors (Xu et al., 2020).
The present study identified a total of 65 organic volatile compounds, including aldehydes, alcohols, ketones, esters, acids, furans, pyrazines, and sulfur-containing compounds in SC breast meat, which agreed with our previous reports (Xu et al., 2020, 2021). The aldehydes, alcohols, and ketones were likely produced by thermal oxidative decomposition of unsaturated fatty acids and lipids, while nitrogen compounds of furans and pyrazines were probably derived from Maillard reactions (Ruiz et al., 2002). Aldehydes are known as key odor compounds of cooked chicken with the characteristics of strong volatility, high concentration, and low threshold. Hexanal, heptenal, and pentanal exhibited strong intensity and were affected by chicken breed. It has been indicated that pentanal has a “woody, fruity aroma”, and a “green, grasslike” aroma associated with hexanal and a “fruity, fatty aroma” associated with certain heptanal (Zeng et al., 2007). In addition, hexanal and heptanal were mainly generated by the oxidation of n-6 polyunsaturated fatty acids, suggesting that they are indicators of lipid oxidation (Hu et al., 2022). Octanal and nonanal, the products of the oxidation of n-9 polyunsaturated fatty acids (Lin et al., 2015), were also observed in SC with higher contents in 817 cross broilers than in yellow-feathered chickens. It should be noted that benzaldehyde was mostly detected in the TE75 group, while it was an important flavor component formed by the Strecher reaction (Baek and Cadwallader, 1997) and suggested to contribute to cardboard-like and musty attributes of SC at higher concentrations (Xu et al., 2020). Chicken age showed a profound effect on the alcohol content in the SC. The dominant alcohols were ethanol, 1-hexanol, and pentanol, among which 1-hexanol had a low odor threshold value mainly derived from the degradation of lipid hydroperoxides (Xu et al., 2020). Ethanol, as one of the volatile spoilage markers, was most frequently observed in the TE75 group, indicating an undesirable smelling of SC (MiksKrajnik et al., 2015). In contrast, a higher content of 1-octen-3-ol, a fatty characteristic of meat flavor, was found in MH75 group, but lower content was found in the TE75 group of boiled chickens. Due to the high threshold and low content detected in SC, the ketones, esters, and nitrogen-containing compounds may exert minor effects on aroma perception (Xu et al., 2020). Furans are generated by the dehydration of carbohydrates, and/or oxidation of fatty acids and are considered vital heterocyclic compounds of meat flavor (Fay and Brevard, 2005). 2-Pentylfuran was mostly found in the 817 cross broiler group, while tetrahydrofuran and 2,5-dimethylfuran were highly detected in the TE group. Moreover, sulfur volatiles were often associated with off-odor flavor and low odor thresholds (Ahn and Lee, 2002), while 2 sulfur-containing compounds, dimethyl sulfide and thiophene were more frequently detected in the TE75 group, indicating an adverse effect on the overall flavor of SC.
In summary, the meat quality including pH, color, product yield, tenderness, textural, and sensorial properties as well as flavor volatile compounds was characterized in SC. Those parameters were affected by chicken breed and slaughter age from principal component analysis. The SC prepared with the yellow-feathered chicken breed, especially Mahuang chicken, exhibited higher product yield, lower shear force, and overall acceptability in comparison to the cross broiler. The slaughter age was shown to have a significant effect on the pH, color, shear force, and textural properties of SC. The relatively young age of yellow-feathered chicken (60 d and 65 d) was suggested to produce desired meat tenderness and textural properties of SC, which was more preferred with high scores by the sensory panel. The volatile compounds, including aldehydes, alcohols, ketones, esters, acids, furans, pyrazines, and sulfur-containing compounds were identified in SC. Mahuang, Tuer, and cross broiler were variably scattered in the PCA plot, while the older-age chicken group was distantly clustered, indicating different characteristic volatiles of SC. Overall, the results of this study suggest that it is feasible to use Mahuang and Tuer chickens for preparing SC and provide valuable information on yellow-feathered chickens for meat processing and industrialization.
ACKNOWLEDGMENTS
This study was supported by China Agriculture Research System of MOF and MARA 560 (CARS-41) and Wens Fifth Five R&D Major Project (WENS-2020-1-ZDZX-007).
DISCLOSURES
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2022.102168.
Appendix. Supplementary materials
REFERENCES
- Abdullah A.Y., M M., Maharmeh H.O., Matarneh S.K., Ishmails M.A.A. Effects of strain on performance, and age at slaughter and duration of post-chilling aging on meat quality traits of broiler. Asian-Austral. J. Anim. 2010;23:1645–1656. [Google Scholar]
- Ahn D.U., Lee E.J. Production of off-odor volatiles from liposome-containing amino acid homopolymers by irradiation. J. Food Sci. 2002;67:2659–2665. [Google Scholar]
- Baek H.H., Cadwallader K.R. Character-impact aroma compounds of crustaceans. Am. Chem. Soc. 1997;674:85–94. [Google Scholar]
- Brewer V.B., Kuttappan V.A., Emmert J.L., Meullenet J.F.C., Owens C.M. Big-bird programs: effect of strain, sex, and debone time on meat quality of broilers. Poult. Sci. 2012;91:248–254. doi: 10.3382/ps.2011-01705. [DOI] [PubMed] [Google Scholar]
- Deng S.L., Li M., Wang H.H., Xu X.L., Zhou G.H. Enhancement of the edible quality and shelf life of soft-boiled chicken using MAP. Food Sci. Nutr. 2020;8:1596–1602. doi: 10.1002/fsn3.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclos M.J., Berri C., Bihan-Duval E.L. Muscle growth and meat quality. J. Appl. Poult. Res. 2007;16:107–112. [Google Scholar]
- Dyubele N.L., Muchenje V., Nkukwana T.T., Chimonyo M. Consumer sensory characteristics of broiler and indigenous chicken meat: a South African example. Food Qual. Prefer. 2010;217:815–819. [Google Scholar]
- El-Senousey H.K., Wang W.W., Wang Y.B., Fan Q.L., Fouad A.M., Lin X.J., Guo Z.Y., Li L., Jiang S.Q. Dietary metabolizable energy responses in yellow-feathered broiler chickens from 29 to 56 d. J. Appl. Poult. Res. 2019;28:974–981. [Google Scholar]
- FAS GAIN/USDA. 2020. Global agricultural information network (GAIN). USDA Foreign Agricultural Service. Accessed Dec. 5, 2021.https://www.fas.usda.gov/databases/global-agricultural-information-network-gain.
- Fay L.B., Brevard H. Contribution of mass spectrometry to the study of the maillard reaction in food. Mass Spectrom. Rev. 2005;24:487–507. doi: 10.1002/mas.20028. [DOI] [PubMed] [Google Scholar]
- Feng Y.C., Cai Y., Fu X., Zheng L., Xiao Z.B., Zhao M.M. Comparison of aromaactive compounds in broiler broth and native chicken broth by aroma extract dilution analysis (AEDA), odor activity value (OAV) and omission experiment. Food Chem. 2018;265:274–280. doi: 10.1016/j.foodchem.2018.05.043. [DOI] [PubMed] [Google Scholar]
- Fletcher D.L. Poultry meat quality. Worlds Poult. Sci. J. 2002;58:131–145. [Google Scholar]
- Hu Y.Y., Zhang L., Wen R.X., Chen Q., Kong B.H. Role of lactic acid bacteria in flavor development in traditional Chinese fermented foods: a review. Crit. Rev. Food Sci. 2022;62:2741–2755. doi: 10.1080/10408398.2020.1858269. [DOI] [PubMed] [Google Scholar]
- Jaturasitha S., Chaiwang N., Kreuzer M. Thai native chicken meat: an option to meet the demands for specific meat quality by certain groups of consumers; a review. Anim. Prod. Sci. 2016;57:1582–1587. [Google Scholar]
- Jaturasitha S., Srikanchai T., Kreuzer M., Wicke M. Differences in carcass and meat characteristics between chicken indigenous to northern Thailand (black-boned and Thai native) and imported extensive breeds (Bresse and Rhode Island Red) Poult. Sci. 2008;87:160–169. doi: 10.3382/ps.2006-00398. [DOI] [PubMed] [Google Scholar]
- Jayasena D.D., Ahn D.U., Nam K.C., Jo C. Factors affecting cooked chicken meat flavour: a review. Worlds Poult. Sci. 2013;69:515–526. [Google Scholar]
- Jayasena D.D., Jung S., Kim H.J., Bae Y.S., Yong H.I., Lee J.H., Kim G.J., Jo C. Comparison of quality traits of meat from korean native chickens and broilers used in two different traditional Korean cuisines. Asian-Australas. J. 2013;26:1038–1046. doi: 10.5713/ajas.2012.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katemala S., Molee A., Thumanu K., Yongsawatdigul J. Meat quality and raman spectroscopic characterization of korat hybrid chicken obtained from various rearing periods. Poult. Sci. 2021;100:1248–1261. doi: 10.1016/j.psj.2020.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Toto C., Were L.L. Antioxidant effectiveness of ground roasted coffee in raw ground top round beef with added sodium chloride. LWT- Food Sci Technol. 2015;60:29–35. [Google Scholar]
- Mancini R.A., Hunt M.C. Current research in meat color. Meat Sci. 2005;71:100–121. doi: 10.1016/j.meatsci.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Mehaffey J.M., Pradhan S.P., Meullenet J.F., Emmert J.L., Owens C.M. Meat quality evaluation of minimally aged broiler breast fillets from five commercial genetic strains. Poult. Sci. 2006;85:902–908. doi: 10.1093/ps/85.5.902. [DOI] [PubMed] [Google Scholar]
- MiksKrajnik M.M., Yoon Y., Yuk H. Detection of volatile organic compounds as markers of chicken breast spoilage using HS-SPME-GC/MS-FASST. Food Sci. Biotechnol. 2015;24:361–372. [Google Scholar]
- NRC . 9th rev. ed. National Academy of Science Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- PołTowicz K., Doktor J. Effect of slaughter age on performance and meat quality of slow-growing broiler chickens. Ann. Anim. Sci. 2012;12:621–631. [Google Scholar]
- Ruiz J., Garcıa C., Muriel E., Andres A.I., Ventanas J. Influence of sensory characteristics on the acceptability of dry-cured ham. Meat Sci. 2002;61:347–354. doi: 10.1016/S0309-1740(01)00204-2. [DOI] [PubMed] [Google Scholar]
- Sarsenbek A., Wang T., Zhao J.K., Jiang W. Comparison of carcass yields and meat quality between Baicheng-You chickens and Arbor Acres broilers. Poult. Sci. 2013;92:2776–2782. doi: 10.3382/ps.2012-02841. [DOI] [PubMed] [Google Scholar]
- Shi H.B., Shahidi F., Wang J.K., Huang Y., Zou Y., Xu W.M., Wang D.Y. Techniques for postmortem tenderisation in meat processing: effectiveness, application and possible mechanisms. Food Product. Process. Nutr. 2021;3:1–26. [Google Scholar]
- Shvartsburg A.A. Ion mobility spectrometry (IMS) and mass spectrometry (MS) Encyclop. Spectrosc. Spectromet. 2010;23:1140–1148. [Google Scholar]
- Sitz B.M., Calkins C.R., Feuz D.M., Umberger W.J., Eskridge K.M. Consumer sensory acceptance and value of domestic, Canadian, and Australian grass-fed beef steaks. J. Anim. Sci. 2005;83:2863–2868. doi: 10.2527/2005.83122863x. [DOI] [PubMed] [Google Scholar]
- Tallentire C.W., Leinonen I., Kyriazakis I. Breeding for efficiency in the broiler chicken: a review. Agron Sustain Dev. 2016;36:66. [Google Scholar]
- Tang H., Gong Y.Z., Wu C.X., Jiang J., Wang Y., Li K. Variation of meat quality traits among five genotypes of chicken. Poult. Sci. 2009;88:2212–2218. doi: 10.3382/ps.2008-00036. [DOI] [PubMed] [Google Scholar]
- Wang H.H., Qin Y., Li J.H., Xu X.L., Zhou W.G. Edible quality of soft-boiled chicken processing with chilled carcass was better than that of hot-fresh carcass. Food Sci. Nutr. 2019;7:797–804. doi: 10.1002/fsn3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.D., Zhang Y., Kong L.L., Wang Z.X., Bai H., Jiang Y., Bi Y.J., Chang G.B., Chen G.H. Effects of rearing system (floor vs. cage) and sex on performance, meat quality and enteric microorganism of yellow feather broilers. J. Integr Agr. 2021;20:1907–1920. [Google Scholar]
- Wattanachant S., Benjakul S., Ledward D.A. Composition, color, and texture of thai indigenous and broiler chicken muscles. Poult. Sci. 2004;83:123–128. doi: 10.1093/ps/83.1.123. [DOI] [PubMed] [Google Scholar]
- Wideman N., O'Bryan C.A., Crandall P.G. Factors affecting poultry meat colour and consumer preferences - a review. Worlds Poult. Sci. J. 2016;72:353–366. [Google Scholar]
- Wu J.W., Lin Z.T., Chen G.H., Luo Q.B., Nie Q.H., Zhang X.Q., Lou W. Characterization of chicken skin yellowness and exploration of genes involved in skin yellowness deposition in chicken. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.585089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N., Ye J.J., Li L.Y., Wang X., Wang P., Han M., Xu X. Exploration of flavor and taste of soft-boiled chicken at different post-mortem aging time: Based on GC-IMS and multivariate statistical analysis. Food Biosci. 2021;43 [Google Scholar]
- Xu Y., Chen Y.P., Deng S.L., Li C.B., Xu X.L., Zhou G.H., Liu Y. Application of sensory evaluation, GC-ToF-MS, and E-nose to discriminate the flavor differences among five distinct parts of the Chinese blanched chicken. Food Res. Int. 2020;137 doi: 10.1016/j.foodres.2020.109669. [DOI] [PubMed] [Google Scholar]
- Yao W.S., Cai Y.X., Liu D.Y., Chen Y., Li J.R., Zhang M.C., Chen N., Zhang H. Analysis of flavor formation during production of dezhou braised chicken using headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS) Food Chem. 2022;370 doi: 10.1016/j.foodchem.2021.130989. [DOI] [PubMed] [Google Scholar]
- Yarmand M.S., Homayouni A. Quality and microstructural changes in goat meat during heat treatment. Meat Sci. 2010;86:451–455. doi: 10.1016/j.meatsci.2010.05.033. [DOI] [PubMed] [Google Scholar]
- Zeng Z., Zhang H., Chen J.Y., Zhang T., Matsunaga R. Direct extraction of volatiles of rice during cooking using solid-phase microextraction. Cereal Chem. 2007;84:423–427. [Google Scholar]
- Zheng H.B., Xiong G.Y., Han M.Y., Deng S.L., Xu X.L., Zhou G.H. High pressure/thermal combinations on texture and water holding capacity of chicken batters. Innov. Food Sci. Emerg. 2015;30:8–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.