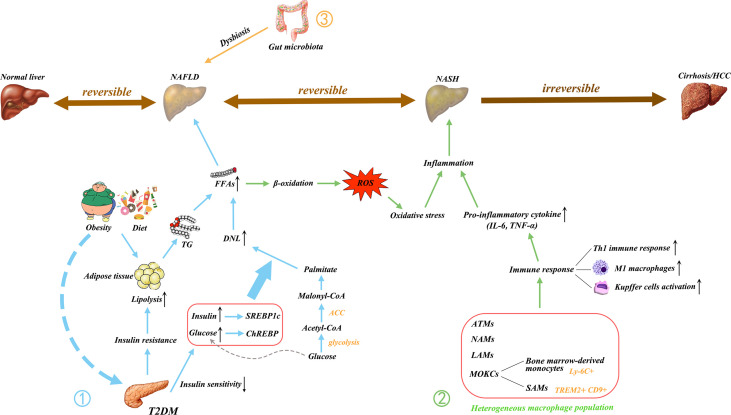
Figure 1.
Main pathogenic mechanism of NAFLD. NAFLD is a multifactorial disease; Obesity, diet, and insulin resistance are linked to pathogenesis. ① The increase in free fatty acids (FFAs) is essential to the development of NAFLD. The FFAs produced by the lipolysis of TG in adipose tissue are delivered to the liver, resulting in hepatic steatosis. T2DM causes IR and decreases insulin sensitivity, thus triggering an increase in DNL, which is another major contributor to the increase in FFAs. The excessive delivery of FFAs to the liver upregulates β-oxidation and promotes lipotoxicity, leading to oxidative stress. ② In addition, the activated immune cells secrete pro-inflammatory cytokines. These processes initiate hepatic inflammation and contribute to the transition from NAFLD to NASH. ③ Gut microbiota dysregulation has also been implicated in the pathogenesis of NAFLD. Gut dysbiosis disrupt the intercellular tight junctions, thus allowing the entry of bacterial lipopolysaccharide into the systemic circulation and increasing liver input. Ultimately, it results in liver exposure to inflammatory mediators. NAFLD, nonalcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; HCC, hepatocellular carcinoma; FFAs, free fatty acids; TG, triglyceride; T2DM, type 2 diabetes mellitus; IR, insulin resistance; DNL, de novo lipogenesis; LPS, lipopolysaccharide; ROS, reactive oxygen species; IL, interleukin; TNF, tumor necrosis factor; Th1, T helper type 1; M1, classically activated macrophage; ATMs, adipose tissue macrophages; NAMs, NASH-associated macrophages; LAMs, lipid-associated macrophages; MOKC, monocyte-derived KCs; SAMs, scar-associated macrophage; TREM2, triggering receptors expressed on myeloid cells 2; CD, cluster of differentiation; CoA, coenzyme A; ACC, acetyl-CoA carboxylase; SREBP1c, sterol regulatory element binding protein 1C (SREBP1c); ChREBP, carbohydrate regulatory element binding protein.