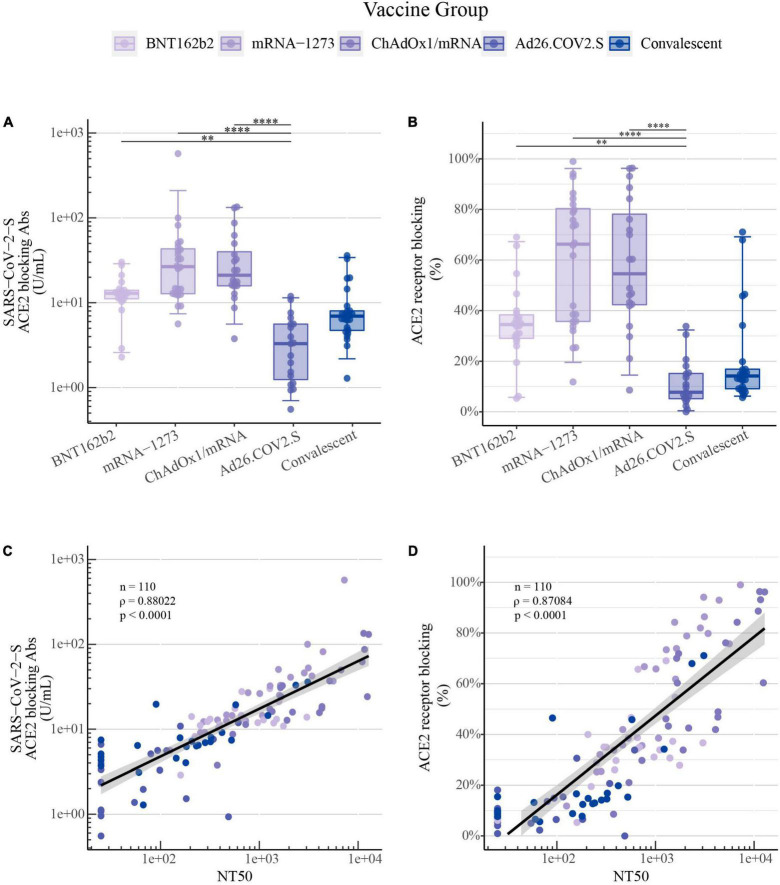
FIGURE 3.
ACE2 competition assay as a surrogate for quantifying COVID-19 vaccine-induced antibody neutralization capacity: (A) SARS-CoV-2-S ACE2 receptor-blocking antibodies in U/mL and (B) ACE2 receptor blocking in percentage for SARS-CoV-2-S wt induced by primary COVID-19 vaccination with BNT162b2 (n = 22), mRNA-1273 (n = 24), ChAdOx1/mRNA (n = 20) or Ad26.COV2.S (n = 19) quantified by the MSD platform (serum 1:100). Data from convalescent comparators (n = 25) are also displayed, but are not included in the statistical analysis. The boxplots present the lower quartile, median and upper quartile, and the error bars indicate 95% CI. P-values were indicated as follows: **p < 0.01 and ****p < 0.0001. (C) Spearman’s correlation between SARS-CoV-2-S wt NT50 values quantified by the pseudovirus neutralization assay and ACE2 receptor-blocking antibodies in U/mL and (D) ACE2 receptor blocking in percentage quantified by the MSD ACE2 competition assay.